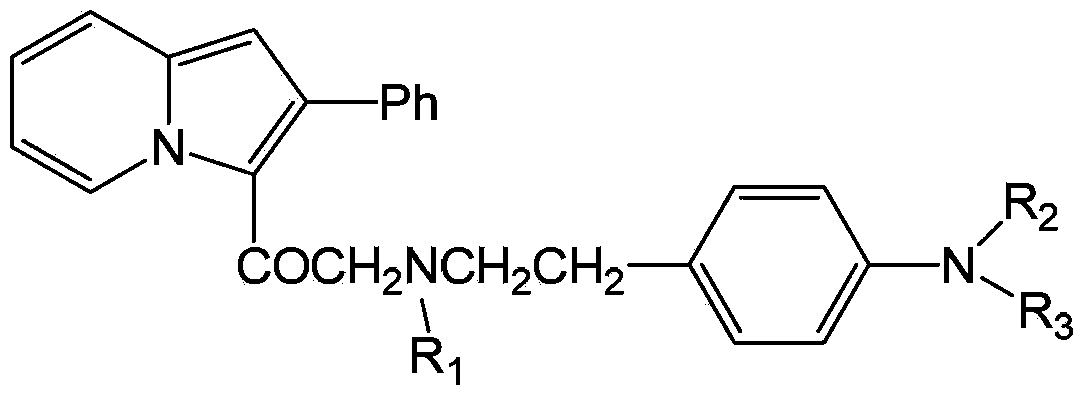
Pyrrocoline formylmethyl p-methane sulfonamide phenylethylamine derivative and its medical application
A technology of indolizine formylmethyl and methanesulfonamide, which is applied in the field of indolizine formylmethyl p-methylsulfonamide phenethylamine derivatives and their medical applications, and can solve the problem of restricting the wide application of Class III antiarrhythmic drugs and other problems to achieve the effect of overcoming side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
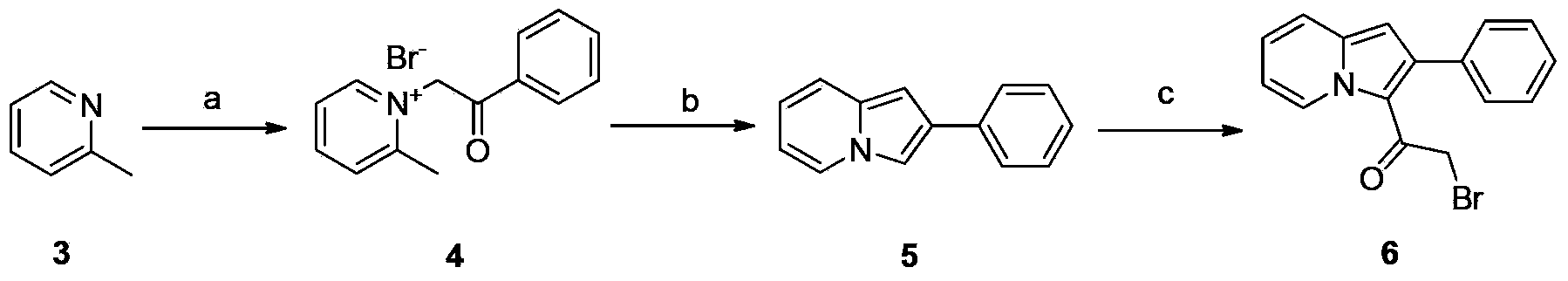
Embodiment 1
[0043] Preparation of 2-phenyl 3-bromoacetyl indolizine (compound 6)
[0044] a) Preparation of N-phenacylmethyl-2-methylpyridinium salt (compound 4)
[0045] 2-Methylpyridine (Compound 3) (4.7g, 0.05mol) and ω-bromoacetophenone (9.9g, 0.05mol) were heated to reflux in 200ml of ethyl acetate for 2 hours, cooled, and the white solid was collected by filtration. After washing with ethyl acetate, 12.1 g of a white solid product was obtained, with a yield of 83%, mp: 215-217°C.
[0046] b) Preparation of 2-phenylindolizine (compound 5)
[0047] Compound 4 (14.6g, 0.05mol) was dissolved in 150ml of water and 80ml of dichloromethane, adding K 2 CO 3 (13.8g, 0.1mol), stirred at room temperature for 1 hour, separated the organic layer, washed once with water, anhydrous MgSO 4 Dry and concentrate to obtain pale yellow crystals (6.9g, 72%), mp: 193-194°C.
[0048] c) Synthesis of 2-phenyl 3-bromoacetyl indolizine (compound 6)
[0049] 2-Phenylindolizine (compound 5) (1.9g, 0.01mol...
Embodiment 2
[0051] Preparation of β-(4-nitrophenyl)-ethylamine hydrobromide (hydrobromide of compound 10)
[0052] a) Preparation of 1-(4-nitrophenyl)-2-(N-acetylamino)ethane (compound 9)
[0053] In 100ml (0.796mol) of β-phenylethylamine (compound 7), add 76ml (0.804mol) of acetic anhydride dropwise under cooling in an ice bath, keep the temperature at 40-45°C, and stir for 2 hours after dropping.
[0054] In a mixture of 150ml (2.81mol) of concentrated sulfuric acid and 150ml (3.33mol) of nitric acid, add the above standby solution dropwise at 20°C, continue to react for 2h after dropping, pour into ice water, extract three times with ethyl acetate, and combine the ester layers , washed with water, anhydrous Na 2 SO 4 Dry, concentrate to dryness, recrystallize from acetone 2 water (recrystallize using a mixed solvent of acetone and water, volume ratio 2:1), and obtain 76g of white needle crystals, yield 46.0%, mp138-140℃.
[0055] b) Preparation of β-(4-nitrophenyl)-ethylamine hydrob...
Embodiment 3
[0058] Preparation of N-(2-phenyl-3-indolizine formylmethyl)-p-nitrophenylethylamine (compound 11)
[0059] 2g (0.012mol) p-nitrophenylethylamine (compound 10), 2g (0.014mol) potassium carbonate, 20ml acetonitrile, heated and stirred under reflux for 20 minutes, cooled to room temperature, slowly added dropwise 2.5g (0.008mol) dissolved in 20ml acetonitrile ) 2-phenyl-3-bromoacetylindolizine (compound 6), stirred and reacted for 2 hours, poured the reaction solution into a large amount of ice water, allowed to stand, and filtered with suction to obtain 3.5 g of a yellow solid (wet product). Developing agent: petroleum ether: ethyl acetate = 1:1, R f =0.33. IR(KBr,cm -1 ):3066,2911,1615,1511,1410,742,702; 1 H NMR (CDCl 3 ,300MHz)δ(ppm):2.79(t,2H,J=6.7Hz,- CH 2 CH 2 N),2.88(t,2H,J=6.7Hz,-CH 2 CH 2 N),3.41(s,2H,-CO CH 2 -),6.49(s,1H,ArH),6.92(t,1H,J=6.9Hz,ArH),7.20-7.55(m,10H,ArH and-NH-),8.12(d,2H,J=8.5 Hz,ArH),9.97(d,1H,J=7.1Hz,ArH);ESI-MS m / z:400.2[M+H] + ;Anal.C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com