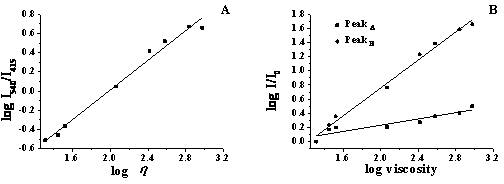
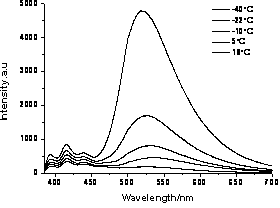
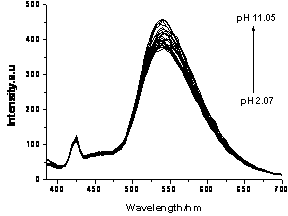
Naphthalimide fluorochrome and its preparation and application
A technology of fluorescent dyes and naphthalimide, applied in the directions of naphthalene dicarboxamide dyes/phthalimide dyes, luminescent materials, fluorescence/phosphorescence, etc., to achieve the effects of mild reaction conditions, strong practical application value, and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the preparation method of compound I
[0041] Heat 5g (0.018mol) of 4-bromo-1,8-naphthalene anhydride and 300ml ethanol to 78°C, dissolve and cool to 50°C, add 2.63mL (0.018mol) of n-butylamine dropwise, reflux for 8 hours, and cool to precipitate The solid was filtered to obtain compound I, 5 g (yield 83.3%).
Embodiment 2
[0042] Embodiment 2: the preparation method of compound II
[0043]
[0044] Mix 3.31g (0.01mol) of compound I and 1.61g (0.02mol) of N-methylethanolamine in 20ml of ethylene glycol methyl ether, add 2.78ml (0.02mol) of triethylamine under magnetic stirring, and react at 124°C for 12 hours. After cooling to room temperature, the reaction solution was added to ice water, a large amount of product was precipitated, and 2.45 g of pure product was obtained by passing through the column, with a yield of 75.15%. NMR and mass spectrometry data for product structure identification: 1 H NMR (400 MHz, CDCl3) δ 8.60 (d, J = 7.3 Hz, 1H), 8.51 (d, J = 8.2 Hz, 2H), 7.71 (d, J = 8.5 Hz, 1H), 7.32 – 7.18 (m , 4H), 4.21 – 4.11 (m, 2H), 3.74 (t, J = 6.9 Hz, 2H), 3.59 (t, J = 7.0 Hz, 2H), 3.10 (s, 3H), 1.77 – 1.65 (m, 2H), 1.45 (m, J = 7.5 Hz, 2H), 0.97 (t, J = 7.4 Hz, 3H).
[0045] HRMS-ESI: m / z calcd for [M + H] + C 19 h 21 N 2 o 2 Br ,389.0786; found,389.085.
Embodiment 3
[0046] Embodiment 3: the preparation method of compound III
[0047]
[0048] 1.63g (0.005mol) of compound II was dissolved in 20ml of dichloromethane, and 0.57ml (0.006mol) of phosphorus tribromide was added dropwise under ice cooling, and reacted at 0°C for 8 hours. The reaction solution was poured into ice water and continued to stir for 0.5 hours, washed with water three times to collect the organic phase, dried over anhydrous magnesium sulfate, filtered and spin-dried to obtain a crude product. After passing through the column (petroleum ether: ethyl acetate = 4:1 as the eluent), 1.38 g of pure product was obtained (71.1% yield). NMR and mass spectrometry data for product structure identification: 1 H NMR (400 MHz, CDCl3) δ 8.60 (d, J = 7.3Hz, 1H), 8.51 (d, J = 8.2 Hz, 2H), 7.71 (d, J = 8.5 Hz, 1H), 7.32 – 7.18 (m , 4H), 4.21 – 4.11 (m, 2H), 3.74 (t, J = 6.9 Hz, 2H), 3.59 (t, J = 7.0 Hz, 2H), 3.10 (s, 3H), 1.77 – 1.65 (m, 2H), 1.45 (m, J = 7.5 Hz, 2H), 0.97 (t, J = ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com