Method for synthesizing hydroxyapatite crystals
A technology for synthesizing hydroxyapatite and hydroxyapatite, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve problems such as irregular crystal shape, difficult crystal plane control, and uneven particle size, and achieve The effect of low cost, convenient operation and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
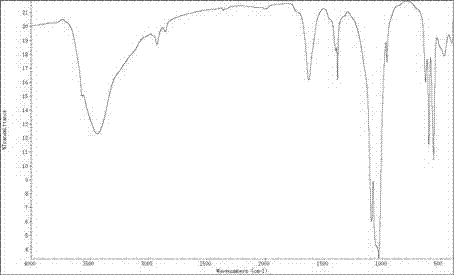
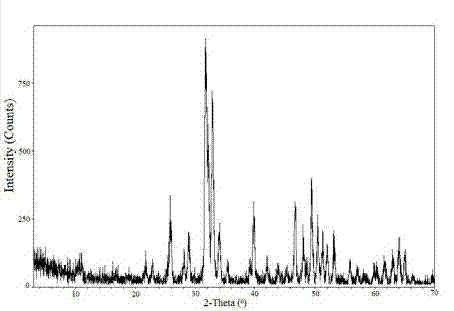
[0030] Ca(NO 3 ) 2 4H 2 O makes it dissolve, disodium edetate and Ca(NO 3 ) 2 The molar ratio of the stearic acid is 1:2, and the mass fraction of stearic acid is 5%. Then (NH 4 ) 2 HPO 4 The solution was added to the above solution. Ca(NO 3 ) 2 4H 2 O and (NH 4 ) 2 HPO 4 The molar ratio is 5:3. The pH value of the reaction solution was adjusted to 9 with ammonia water, and stirred at constant temperature for 2 hours. Afterwards, the solution was added into a stainless steel hydrothermal synthesis kettle lined with a polytetrafluoroethylene liner, and reacted at 140° C. for 20 hours to obtain a white precipitate. The white precipitate was suction filtered, washed, and freeze-dried for 24 hours to obtain the target product. figure 1 For the infrared spectrogram of the hydroxyapatite prepared in this embodiment, in the IR spectrum, 3568cm ?1 and 628 cm ?1 The peak is the characteristic peak of hydroxyl (OH), 871 cm ?1 is HPO 4 2? peak at 1400 cm ?1 and 1500...
Embodiment 2
[0032] Ca(NO 3 ) 2 4H 2 O makes it dissolve, disodium edetate and Ca(NO 3 ) 2 The molar ratio of the stearic acid is 1:2, and the mass fraction of stearic acid is 10%. Then (NH 4 ) 2 HPO 4 The solution was added to the above solution. Ca(NO 3 ) 2 4H 2 O and (NH 4 ) 2 HPO 4 The molar ratio is 5:3. The pH value of the reaction solution was adjusted to 10 with ammonia water, and stirred at constant temperature for 2 hours. Afterwards, the solution was added to a stainless steel hydrothermal synthesis kettle lined with a polytetrafluoroethylene liner, and reacted at 140° C. for 16 hours to obtain a white precipitate. The white precipitate was suction-filtered, washed, and freeze-dried for 24 hours to obtain the target product.
Embodiment 3
[0034] Ca(NO 3 ) 2 4H 2 O makes it dissolve, disodium edetate and Ca(NO 3 ) 2 The molar ratio of the stearic acid is 1:1, and the mass fraction of stearic acid is 20%. Then (NH 4 ) 2 HPO4 The solution was added to the above solution. Ca(NO 3 ) 2 4H 2 O and (NH 4 ) 2 HPO 4 The molar ratio is 5:3. The pH value of the reaction solution was adjusted to 11 with ammonia water, and stirred at constant temperature for 2 hours. Afterwards, the solution was added into a stainless steel hydrothermal synthesis kettle lined with a polytetrafluoroethylene liner, and reacted at 120° C. for 20 hours to obtain a white precipitate. The white precipitate was suction-filtered, washed, and freeze-dried for 24 hours to obtain the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com