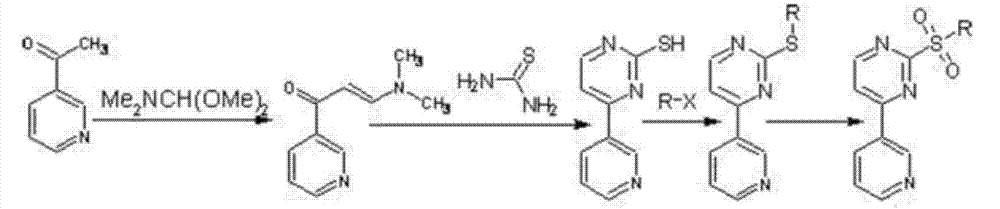
Method for synthesizing N-(5-nitryl-2-methyl pyridyl-3-)-4-(3-pyridyl)-2-pyrilamine and intermediate thereof
A compound, the technology of dimethyl carbonate, which is applied in the field of preparation of pyrimidine derivatives, can solve the problems of scale-up that is difficult to industrialize, complex equipment, and increase the production of impurities in side reactions, so as to achieve increased yield, improved yield and purity, The effect of avoiding the use of more toxic phase transfer catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1- Embodiment 4
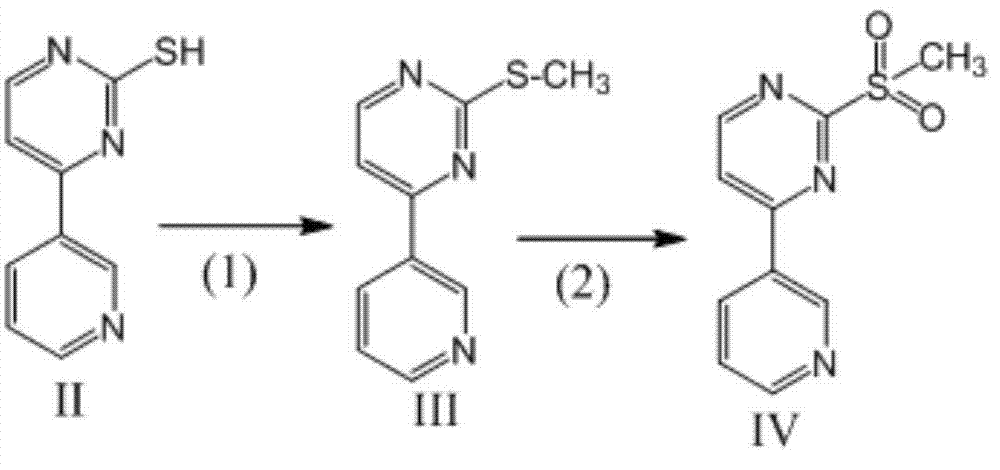
[0026] The preparation of embodiment 1-embodiment 4 compound IV, formula is as follows
[0027]
[0028] Reaction process:
[0029] (1) Put compound II, HMPA, and dimethyl carbonate solution as raw materials into a reaction kettle, stir and dissolve, heat to 60-80°C, and the reaction is complete after 1.5 hours. Concentrate under reduced pressure to 80°C. Take a small amount of reaction solution and dilute it with a large amount of water to obtain a compound III sample and detect the content
[0030] (2) Cool the reaction solution in step (1) directly to -10°C~10°C, adjust the pH to 4-5 with 0.1M hydrochloric acid, then add hydrogen peroxide, react until complete at 20-50°C, add sodium sulfite to consume Excess H 2 o 2 , adjust the pH to 7.5-8, add water with a volume ratio of 20-50 to HMPA, stir while adding, until the precipitate solidifies, filter to obtain a filter cake, wash the filter cake with water and dry to obtain compound IV. The product was weighed and the ...
Embodiment 5
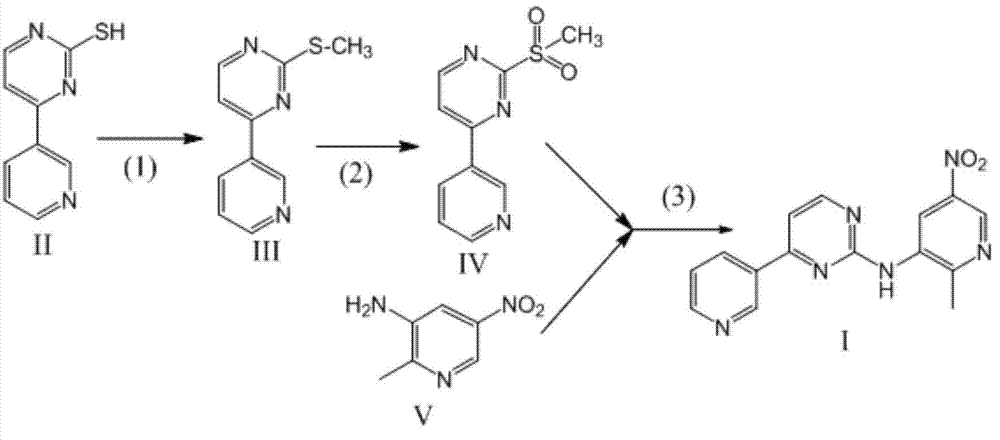
[0047] The preparation of embodiment 5 compound I
[0048] Get the compound IV prepared by the method of Example 1 as a raw material, adopt sodium hydride as a commercially available 60% product, and calculate the amount of feed in terms of sodium hydride
[0049] The preferred process of the step (3) is as follows: add compound IV and compound V with a molar ratio of 1:1.5 into the reactor, add DMF with a volume weight ratio of 8 to compound IV, stir and dissolve, then cool to -10°C and keep at Add sodium hydride () in batches at this temperature, and the molar ratio of added sodium hydride to compound IV is 2:1. After adding sodium hydride, keep the temperature at 20-30°C until the reaction is complete to obtain compound I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com