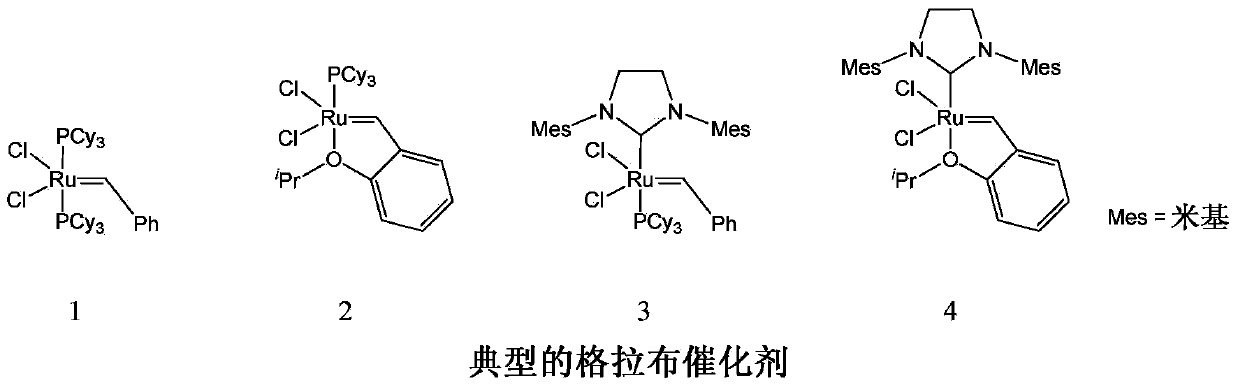
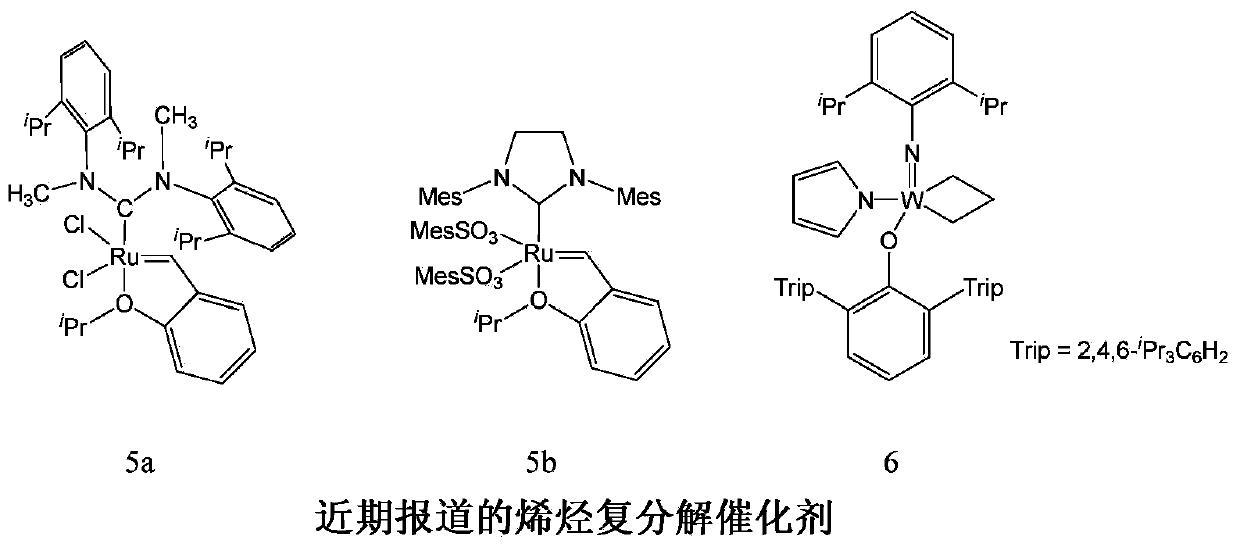
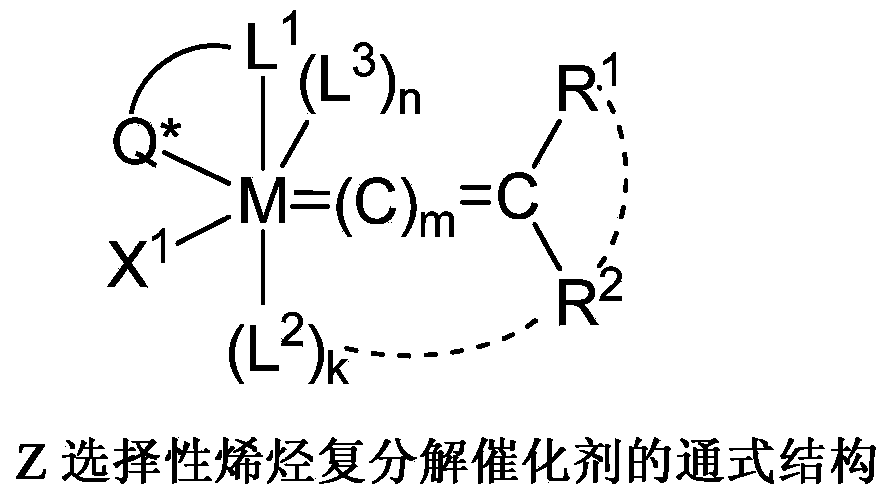
Z-selective olefin metathesis catalysts and their synthetic procedure
一种复分解催化剂、烯烃的技术,应用在催化剂在Z-选择性烯烃复分解反应中的用途领域,能够解决限制用途、多合成步骤反应条件等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Preparation of C-H-activated catalyst complexes from Ru-complex 4
[0136] By making (H 2 IMes)RuCl 2 [=CH-o-(O i Pr)C 6 h 4 ](4) and 2 equivalents of RCOOAg (R= t Bu, PhMe 2 C) reaction, obtain metal cyclization compound {[2-(CH 2 )-4,6-Me 2 (C 6 h 2 )](C 3 N 2 h 4 )-(Mes)}Ru(OCOR)[=CH-o-(O i Pr)C 6 h 4 ](R= t Bu(7a), PhMe 2 C(7b)) (Scheme 4), an air stable dark green solid. In this reaction, the disubstituted complex (8) was also observed at early reaction times. Subsequently, the C-H bond activates the methyl group of the mesityl group in the NHC ligand and forms the corresponding carboxylic acid, providing 7. The molecular structures of 7a and 7b were confirmed by X-ray crystallography. Such as Figure 4 with 5 Both 7a and 7b have 6-membered chelates formed from ruthenium and NHC ligands, as shown in .
[0137] Option 4
[0138]
[0139] The representative characterization data of complex 7a are as follows:
[0140] 1 HNMR (500MHz, C 6 D...
Embodiment 2
[0142] Preparation of C-H-activated catalyst complexes from Ru-complex 9
[0143] In the same manner as Scheme 4, (H 2 IMes)Ru(OTf) 2 [=CH-o-(O i Pr)C 6 h 4 ](9) (prepared as described in Krause, J.O.; Nuyken, O.; Wurst, K.; Buchmeiser, M.R. Chem. Eur. J. 2004, 10, 777) provides chelation in the reaction with the corresponding sodium salt Complexes {[2-(CH 2 )-4,6-Me 2 (C 6 h 2 )](C 3 N 2 h 4 )(Mes)}Ru(OCOR)[=CH-o-(O i Pr)C 6 h 4 ](R= t Bu(7a), Ph 2 MeC(7c), Ph 3 C(7d)) (Scheme 5). The products are all air-stable solids. In these reactions, the formation of the disubstituted complex (8) and subsequent formation of the carboxylic acid in the early stages of the reaction was also observed.
[0144] Option 5
[0145]
Embodiment 3
[0147] Preparation of C-H-activated catalyst complexes from Ru-complex 10
[0148] By making the (H 2 IMesDipp)RuCl 2 [=CH-o-(O i Pr)C 6 h 4 ](10) reacted with silver pivalate to obtain {[2-(CH 2 )-4,6-Me 2 (C 6 h 2 )](C 3 N 2 h 4 )(Dipp)}Ru(OCO t Bu)[=CH-o-(O i Pr)C 6 h 4 ](11) (Scheme 6), an air-stable dark green solid. During this reaction, disubstituted complex (12) was formed without any observed complex originating from activation of the C—H bond in the 2,6-diisopropylphenyl group. The crystal structure of 11 determined by X-ray crystallography ( Image 6 ) shows a 6-membered chelate and clearly shows that the methyl group of the mimidyl group in the NHC ligand has undergone C-H bond activation.
[0149] Option 6
[0150]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com