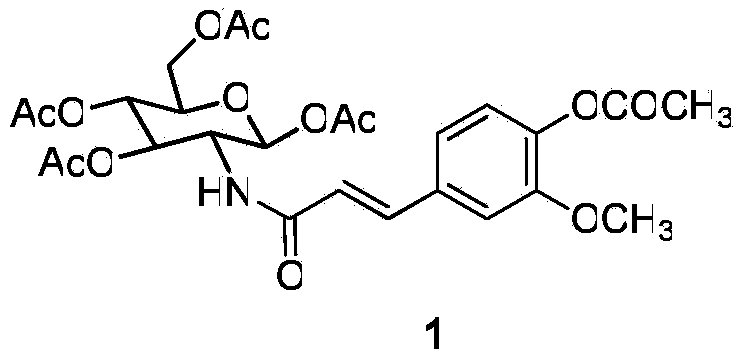
N-(2-deoxy-lactose-2-group)-3-(substituted phenyl) acrylamide and medical application thereof
A representative and pharmaceutical technology, applied in the field of N--3-acrylamide derivatives (I), inhibiting the proliferation of vascular endothelial cells and the formation of new blood vessels in the allantoic membrane of chicken embryos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
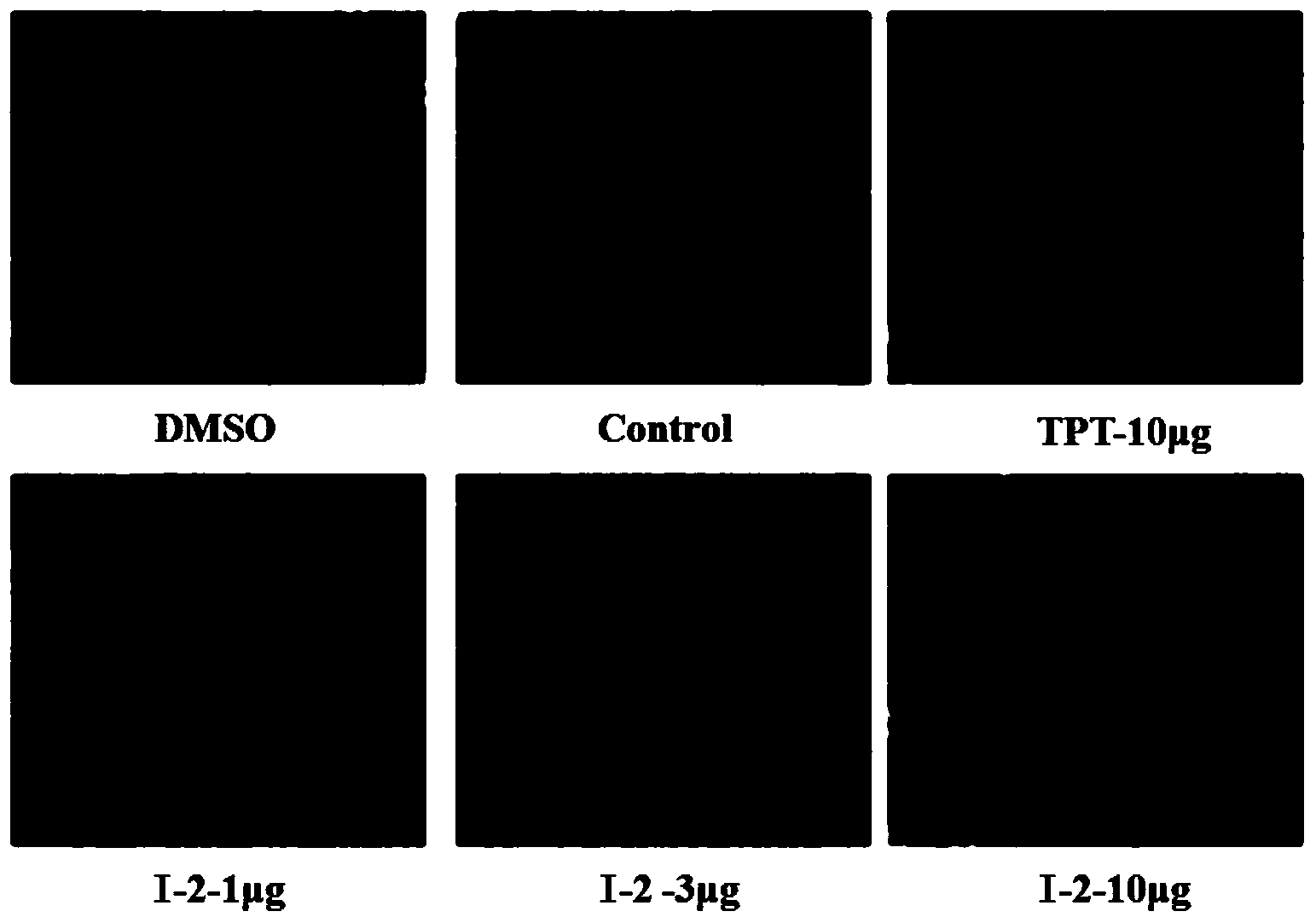
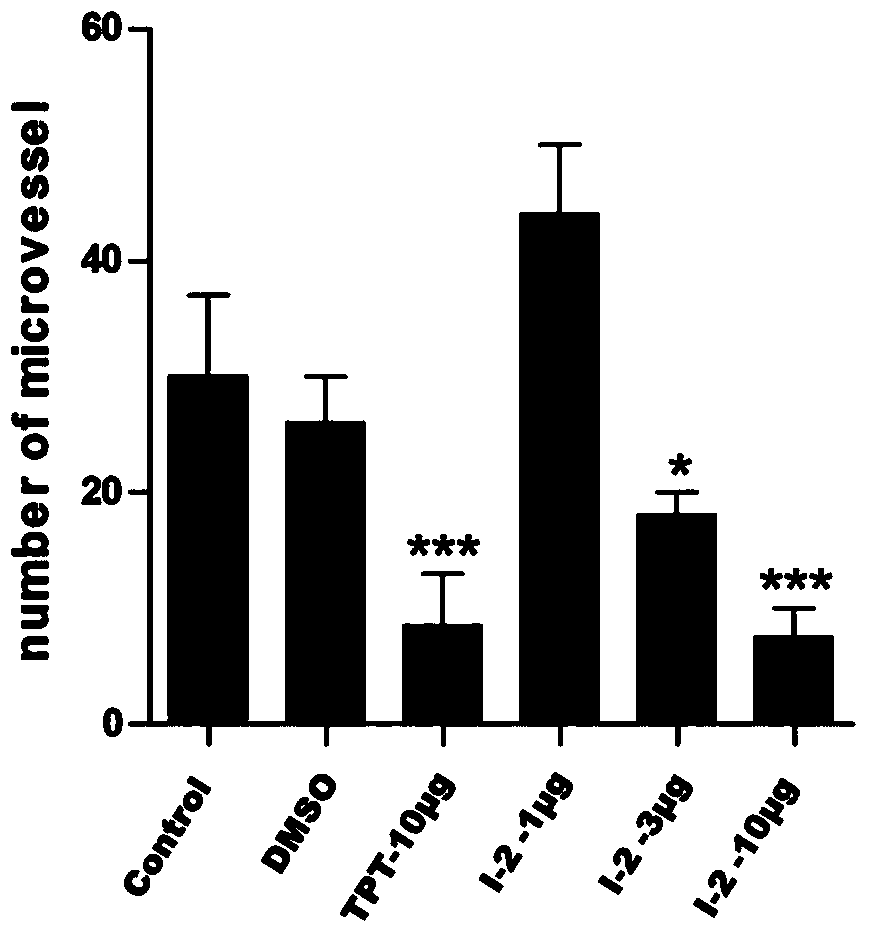
Image
Examples
Embodiment 1
[0042] 1,3,6-Tri-O-acetyl-4-O-(2,3,4,6-tetra-O-acetyl-β-D-galactosyl)-2-amino-2-deoxy- Synthesis of β-D-glucose p-toluenesulfonate (6)
[0043] Hexa-O-acetyl lactose (2)
[0044]Add lactose (5g, 0.0146mol) to a 50mL three-necked flask, suspend in 13.4g (0.13mol, 12.4mL) acetic anhydride, stir electromagnetically at room temperature, keep warm in a water bath, and slowly add (5.0g, 3.5mL) 31% HBr / HAc dropwise , after stirring for 4 h, (20.0 g, 14.0 mL) 31% HBr / HAc was added dropwise and stirred overnight. Pour the reaction solution into 50mL aqueous solution of 0.91g copper sulfate pentahydrate and 75mL acetic acid solution containing 27.4g sodium acetate, stir vigorously for 2h, and keep the inner temperature of about 20°C in the water bath. After filtration, the filter cake was washed successively with ethyl acetate (500 mL), water (500 mL), the organic layer was washed with saturated sodium bicarbonate (300 mL×3), saturated sodium chloride (300 mL×3), and dried over anhydr...
Embodiment 2
[0063] N-[1,3,6-tri-O-acetyl-4-O-(2,3,4,6-tetra-O-acetyl-β-D-galactosyl)-2-deoxy-β -D-glucosyl]-3-(3,4-diacetylphenyl)-2-acrylamide (I-1)
[0064] Suspend 3,4-diacetoxycinnamic acid (0.33 g, 1.25 mmol) in 2 mL of chloroform, slowly add 1 mL of thionyl chloride dropwise under ice-cooling, and stir at room temperature for 1 h. The solvent was removed under reduced pressure to obtain a yellow solid, which was directly put into the next reaction without further purification.
[0065] Compound 6 (1.0 g, 1.24 mmol) was dissolved in 15 mL of dichloromethane, and a solution of sodium carbonate (0.3 g, 2.8 mmol) in water (15 mL) was added with stirring. Dissolve freshly prepared 3,4-diacetoxycinnamoyl chloride in 10 mL of dichloromethane, slowly drop it into the above two-phase solution, stir at room temperature overnight, separate the organic layer, and wash the water layer with dichloromethane (10 mL × 1) extraction, the organic phases were combined and then washed with saturated s...
Embodiment 3
[0071] N-[1,3,6-tri-O-acetyl-4-O-(2,3,4,6-tetra-O-acetyl-β-D-galactosyl)-2-deoxy-β -D-glucosyl]-3-(2,4-chlorophenyl)-2-acrylamide (I-2)
[0072] Suspend 2,4-dichlorocinnamic acid (0.14g, 0.62mmol) in 0.5mL of thionyl chloride, heat under reflux and stir for 2h. The solvent was removed under reduced pressure to obtain a white solid, which was directly put into the next reaction without further purification.
[0073] Compound 6 (0.50g, 0.62mmol) was dissolved in 7mL CH 2 Cl 2 , a solution of sodium carbonate (0.15 g, 1.4 mmol) in water (7 mL) was added with stirring. Dissolve the freshly prepared acid chloride in 4 mL CH 2 Cl 2 , slowly drop into the above two-phase solution, and stir at room temperature for 12h. Transfer the reaction solution into a separatory funnel, separate the organic layer, extract the aqueous layer with dichloromethane (10mL×1), and wash the organic phase with saturated sodium bicarbonate solution (10mL×2) and saturated sodium chloride solution succ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com