Method for preparing angiotensin synthesis receptor based on blotting technology and prepared angiotensin synthesis receptor
An angiotensin and technology technology, which is applied in the field of preparing angiotensin synthesis receptors based on imprinting technology, can solve the problems of complex thermodynamic factors of biological macromolecular structures, limited successful examples of polypeptides and proteins, and difficulty in obtaining molecularly imprinted polymers, etc. Achieve high blotting effect, high affinity and selectivity, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
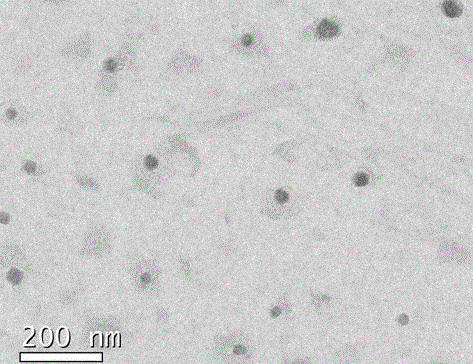
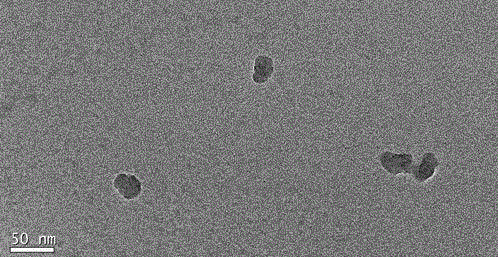
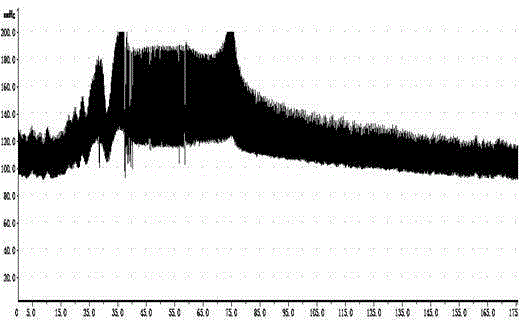
Image
Examples
Embodiment 1
[0030] 0.0045g of the template molecule, namely the N-terminal tripeptide derivative template of angiotensin II (Asp-Arg-Val-NHC 2 h 5 ), 0.098 mmol of acrylic acid, 0.156 mmol of N-isopropylacrylamide were dissolved in 50 mL of distilled water to obtain solution 1. 0.065 mmol of N-tert-butylacrylamide was dissolved in 1 mL of absolute ethanol to obtain solution 2, and solution 2 was mixed into the above solution In 1, solution 3 was obtained after shaking for 4 hours. Add 1 mg of N, N-methylene bisacrylamide and 10 mg of sodium dodecyl sulfate to the above solution 3, pass nitrogen gas for 30 minutes, add 30 mg of initiator ammonium peroxosulfate, 15 μL of N, N, N, N-tetramethyl base diethylamine, sealed, and reacted under nitrogen atmosphere at room temperature for 24 hours. Transfer the reaction solution into a dialysis bag (MWCO10000), and perform dialysis in a large amount of distilled water for four days, changing the water at least twice a day to remove unreacted mono...
Embodiment 2
[0034] 0.0045g of the template molecule, namely the N-terminal tripeptide derivative template of angiotensin II (Asp-Arg-Val-NHC 2 h 5 ), 0.098 mmol of acrylic acid, 0.156 mmol of N-isopropylacrylamide were dissolved in 50 mL of distilled water to obtain solution 1. 0.065 mmol of N-tert-butylacrylamide was dissolved in 1 mL of absolute ethanol to obtain solution 2, and solution 2 was mixed into the above solution In 1, solution 3 was obtained after shaking for 4 hours. Add 1 mg of N, N-methylene bisacrylamide and 10 mg of sodium dodecyl sulfate to the above solution 3, pass nitrogen gas for 30 minutes, add 30 mg of initiator ammonium peroxosulfate, 15 μL of N, N, N, N-tetramethyl base diethylamine, sealed, and reacted under nitrogen atmosphere at room temperature for 24 hours. Transfer the reaction solution into a dialysis bag (MWCO10000), and perform dialysis in a large amount of distilled water for four days, changing the water at least twice a day to remove unreacted mono...
Embodiment 3
[0040] 0.0045g of the template molecule, namely the N-terminal tripeptide derivative template of angiotensin II (Asp-Arg-Val-NHC 2 h 5 ), 0.098 mmol of acrylic acid, 0.156 mmol of N-isopropylacrylamide were dissolved in 50 mL of distilled water to obtain solution 1. 0.065 mmol of N-tert-butylacrylamide was dissolved in 1 mL of absolute ethanol to obtain solution 2, and solution 2 was mixed into the above solution In 1, solution 3 was obtained after shaking for 4 hours. Add 1 mg of N, N-methylene bisacrylamide and 10 mg of sodium dodecyl sulfate to the above solution 3, pass nitrogen gas for 30 minutes, add 30 mg of initiator ammonium peroxosulfate, 15 μL of N, N, N, N-tetramethyl base diethylamine, sealed, and reacted under nitrogen atmosphere at room temperature for 24 hours. Transfer the reaction solution into a dialysis bag (MWCO10000), and perform dialysis in a large amount of distilled water for four days, changing the water at least twice a day to remove unreacted mono...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com