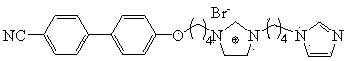
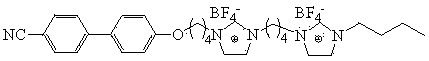Quasi-solid electrolyte for solar cell based on double-imidazole type ionic crystal
A technology of solar cells and ionic crystals, applied in capacitor electrolyte/absorber, photovoltaic power generation, capacitor parts and other directions, can solve the problems affecting the photoelectric conversion efficiency of dye-sensitized solar cells, low conductivity of monoimidazole type ionic crystals, etc. Achieve the effect of improving photoelectric conversion efficiency, high stability, and avoiding leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] This example provides a kind of preparation method of bis-imidazole type ion crystal, as figure 1 shown, including the following steps:
[0022] (1) Add 20mL acetone, 15mmol diiodobutane, 10mmol , 20mmol K 2 CO 3 , under the protection of inert gas at 60°C, reacted for 15 hours and then filtered to obtain the filtrate, which was obtained by rotary evaporation and petroleum ether recrystallization ;
[0023] (2) Add 20mL of acetonitrile, 10mmol of diiodobutane, 25mmol of imidazole, and 35mmol of KOH in sequence in another reaction vessel, react at room temperature for 10 hours and then filter to obtain the filtrate, which is obtained by rotary evaporation and deionized water recrystallization ;
[0024] (3) Add 10mmol Add dropwise to 30mmol in acetone (20mL) solution, condensed and refluxed at 30°C for 10 hours, removed the acetone by rotary evaporation, washed three times with ethyl acetate and diethyl ether, and dried to obtain ;
[0025] (4) Add 10mmol ...
Embodiment 2
[0027] This example provides a kind of preparation method of bis-imidazole type ion crystal, as figure 1 shown, including the following steps:
[0028] (1) Add 20mL acetone, 15mmol diiodobutane, 10mmol , 20mmol K 2 CO 3 , under the protection of inert gas at 80°C, reacted for 20 hours and then filtered to obtain the filtrate, which was obtained by rotary evaporation and petroleum ether recrystallization ;
[0029] (2) Add 20mL of acetonitrile, 10mmol of diiodobutane, 25mmol of imidazole, and 35mmol of KOH in sequence in another reaction vessel, react at room temperature for 15 hours, and then filter to obtain the filtrate, which is obtained by rotary evaporation and deionized water recrystallization ;
[0030] (3) Add 10mmol Add dropwise to 30mmol in acetone (20mL) solution, condensed and refluxed at 30°C for 10 hours, removed the acetone by rotary evaporation, washed three times with ethyl acetate and diethyl ether, and dried to obtain ;
[0031] (4) Add 10mmol...
Embodiment 3
[0033] This example provides a kind of preparation method of bis-imidazole type ion crystal, as figure 1 shown, including the following steps:
[0034] (1) Add 20mL acetone, 15mmol diiodobutane, 10mmol , 20mmol K 2 CO 3 , under the protection of inert gas at 80°C, reacted for 20 hours and then filtered to obtain the filtrate, which was obtained by rotary evaporation and petroleum ether recrystallization ;
[0035] (2) Add 20mL of acetonitrile, 10mmol of diiodobutane, 25mmol of imidazole, and 35mmol of KOH in sequence in another reaction vessel, react at room temperature for 15 hours, and then filter to obtain the filtrate, which is obtained by rotary evaporation and deionized water recrystallization ;
[0036] (3) Add 10mmol Add dropwise to 30mmol in acetone (20mL) solution, condensed and refluxed at 30°C for 10 hours, removed the acetone by rotary evaporation, washed three times with ethyl acetate and diethyl ether, and dried to obtain ;
[0037] (4) Add 10mmol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com