Doramectin O/W type injection taking water as matrix and preparation method thereof
A doramectin and injection technology, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, and pharmaceutical formulas, can solve the problems of insoluble in water, poor stability, etc., and achieve fluidity Good, reduced pain, good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
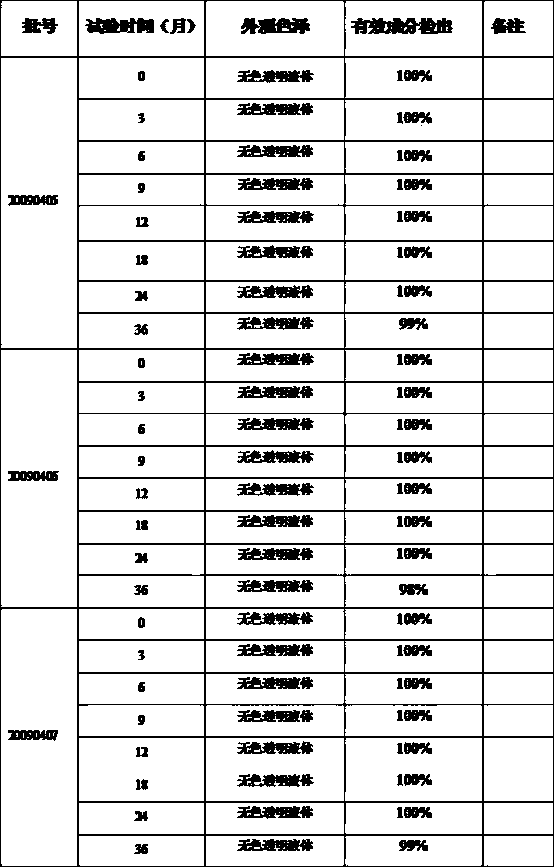
Image
Examples
Embodiment 1
[0027] Embodiment 1: the preparation of the O / W type doramectin injection that content is 0.1%~5%:
[0028] 1) Weigh each drug component: 37.5 grams of OP emulsifier, 12.5 grams of PEG400, 10.0 grams of ethyl oleate, 5.0 grams of doramectin, and place them in a brown triangular flask. Stir for 0.2h until the doramectin is completely dissolved and the system is uniform, transparent and clear.
[0029] 2) Slowly add water for injection into the solution under stirring conditions. With the increase of the amount of water for injection, the solution system first becomes relatively viscous. When the water for injection reaches 25g, the system suddenly becomes thinner and the fluidity increases. It is completely transparent. When the amount of water for injection reaches 35 grams, the content of doramectin is 5% O / W type doramectin injection. At this time, the content of other various excipients is also as high as 60%. The content is high, the toxic and side effects are large, and ...
Embodiment 2
[0032] Embodiment 2: Determination of 1% O / W type doramectin injection
[0033]The content of the prepared 1% O / W doramectin injection was determined by HPLC, using Waters high performance liquid chromatography series; Luna C18 (3μm) 4.6 mm × 150 mm chromatography; Waters510 type infusion Pump; TM480 type fluorescence detector; U6 K type injection valve; AST type data processor; 3800 type printer. The mobile phase is acetonitrile: methanol: glacial acetic acid (64:32:4), flow rate 1 mL / min; column temperature (30.0 ± 0.5) ℃; excitation wavelength 383 nm, emission wavelength 447 nm, injection volume: 20 μl.
[0034] Preparation of reference substance solution: Accurately weigh doramectin reference substance: 0.0100 g, dissolve it in mobile phase and configure it to a concentration of 100.0 μg / ml as a stock solution. Precisely measure 0.2, 0.6, 1.0, 3.0, 6.0, 10.0 μl of the above stock solutions, put them in 10ml volumetric flasks respectively, add mobile phase to constant vol...
Embodiment 3
[0038] Embodiment 3: acute toxicity test result
[0039] (1) Acute toxicity test results of 0.1% O / W doramectin injection
[0040] 30 Kunming mice and 30 Wistar rats (clean grade), male and female, were purchased from the Experimental Animal Center of Lanzhou University, license number: SCXK (Gan) 2009-0004; After intraperitoneal injection of 0.1% O / W type doramectin injection with the content of doramectin original drug (10mg / kg) body weight dose, continuous observation for 7 days, no toxic and side effects were found, and the large and small mice had smooth coat and moved freely , normal diet, and no death during the test period. The test results show that 0.1% O / W doramectin injection has the maximum tolerated dose of LD for intraperitoneal injection in mice and mice. 0 That is to say, if the dose is greater than 10g / kg body weight without death, then its half lethal dose LD 50 The value must be greater than the dose, that is, greater than 10g / kg body weight. Therefore, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com