Dipeptidyl peptidase inhibitor and vitamin-B pharmaceutical composition and application thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
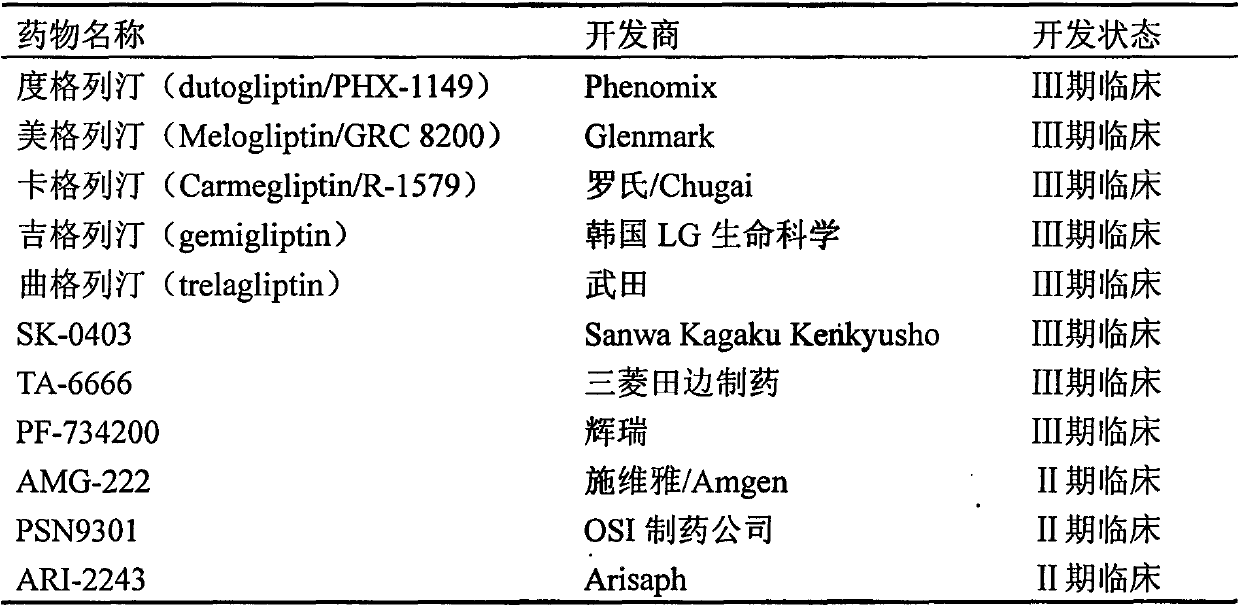
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 prepares compound sitagliptin / folic acid sheet (1000 pieces amount)
[0034]
[0035] Preparation method: pass the raw and auxiliary materials through a 80-mesh sieve, and dry for later use. Take the prescribed amount of folic acid and sitagliptin and mix them uniformly according to the method of equal increase, weigh the prescribed amount of microcrystalline cellulose, low-substituted hydroxypropyl cellulose (L-HPC), sodium carboxymethyl starch, and mix them with the raw materials The powder is fully mixed according to the method of equal increase, and an appropriate amount of 5% povidone 95% ethanol solution is added to make a soft material, granulated with a 20-mesh sieve, dried at 50°C for about 6 hours, and granulated with a 20-mesh sieve to control the water content of the granules. 2-3%, the dried granules are uniformly mixed with the prescribed amount of magnesium stearate, the intermediate is tested, and pressed into 1000 tablets. Pay attention...
Embodiment 2
[0036] Embodiment 2 prepares compound saxagliptin / folic acid sheet (1000 pieces amount)
[0037]
[0038] Preparation method: pass the raw and auxiliary materials through a 80-mesh sieve, and dry for later use. Take the prescribed amount of folic acid and saxagliptin and mix them uniformly according to the method of equal increase, weigh the prescribed amount of microcrystalline cellulose, lactose, cross-linked polyvinylpyrrolidone, and sodium carboxymethyl starch, and fully mix them with the mixed powder of raw materials. Add an appropriate amount of 10% starch slurry solution to make a soft material, granulate with a 20-mesh sieve, dry at 50°C for about 6 hours, sieve the granules with a 20-mesh sieve, control the water content of the granules to 2-3%, and mix the dried granules with the prescribed amount Glyceryl behenate and magnesium stearate were evenly mixed, the intermediate was tested, and pressed into 1000 tablets. Pay attention to avoiding light during the prepa...
Embodiment 3
[0039] Example 3 Preparation of Compound Vildagliptin / 5-Methyltetrahydrofolate Tablets (1000 Tablets)
[0040]
[0041]
[0042] Preparation method: the auxiliary material is a direct pressing auxiliary material, which is dried for later use. Take the prescribed amount of 5-methyltetrahydrofolate and 10 g of microcrystalline cellulose and mix uniformly according to the method of equal increase to obtain the powder mixing intermediate 1; weigh the remaining microcrystalline cellulose, lactose, and sodium carboxymethyl starch in the prescribed amount , and vildagliptin are fully mixed according to the equal increase method to obtain the mixed powder intermediate 2; the mixed powder intermediate 1 and the mixed powder intermediate 2 are mixed with the prescribed amount of magnesium stearate according to the equal increase method, Obtain the final mixed powder intermediate, detect the mixed powder intermediate, and press into 1000 pieces. Pay attention to avoiding light dur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com