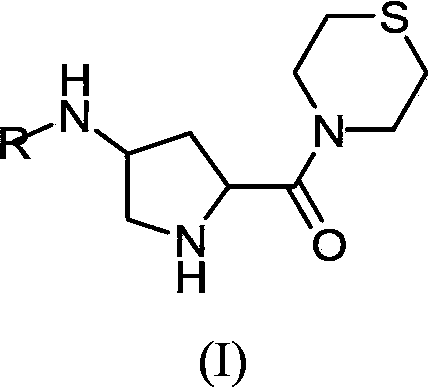
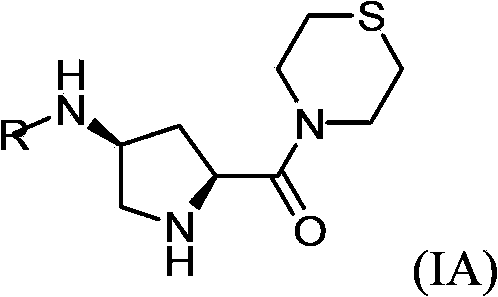
Substituted-pyrrolidinyl-contained thiomorpholine compounds
A technology of compound and alkyl, applied in the field of medicine, can solve problems such as short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0105] Synthetic route of Scheme 1 intermediate 6a
[0106]
[0107] Preparation of the first step (2S, 4R)-N-tert-butoxycarbonyl-4-hydroxypyrrolidine-2-carboxylic acid 2a
[0108] Dissolve 4-OH-L-proline (3.95g, 30.1mmol) in 50mL 1,4-dioxane and 50mL 1mol·L -1 Add (Boc) to the mixed solution of sodium hydroxide solution at 0°C 2 O (7.85g, 36.0mmol), stirred at low temperature for 0.5h, then raised to room temperature and stirred overnight. Concentrate under reduced pressure and evaporate to near dryness, add 50mL water to dissolve, and use 1mol·L -1 Adjust the pH of the HCl solution to 2~3, extract with ethyl acetate, combine the organic layers, wash the organic layer with saturated brine, dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain intermediate 2a, white solid 6.68g, yield About 96.3%. 1 H NMR (D 2 O,300MHz)δ:4.53(br,1H, CH -OH in pyrrolidine),4.42-4.36(m,1H,CHC=O),3.60-3.47(m,2H,CH 2 N in pyrrolidine),2.42-2.35&2.19...
Embodiment 1
[0123]
[0124] Compound 1 4-{[(2S,4S)-4-(piperidin-4-yl-amino)-pyrrol-2-yl]-formyl}thiomorpholine trihydrochloride
[0125]
[0126] Preparation of the first step 4-{[(2S, S)-N-tert-butoxycarbonyl-4-(piperidin-4-yl)amino-pyrrol-2-yl]-formyl}thiomorpholine 1b
[0127] 4-{[(2S,4S)-4-amino-N-tert-butoxycarbonyl-pyrrol-2-yl]-formyl}thiomorpholine 6a (0.15g, 0.48mmol) was mixed with N-Boc-4 -Piperidone (0.098g, 0.49mmol) was dissolved in 10mL of dichloromethane, then a small amount of AcOH was added to adjust the pH of the reaction system to 3-5, stirred at room temperature for 3 hours, the temperature of the reaction system was lowered to below 0°C, and triacetylboron was added Sodium hydride (0.317g, 1.50mmol), raised to room temperature and stirred for 6 hours until the reaction was complete. Add 20 mL of water to the reaction solution, extract with dichloromethane, combine the organic layers, wash with saturated sodium bicarbonate solution and saturated brine, respectiv...
Embodiment 2
[0132]
[0133] Compound 24-{[(2S,4S)-4-(3-Hydroxy-2,2-dimethyl-propylamino)-pyrrol-2-yl]-formyl}thiomorpholine dihydrochloride
[0134]
[0135] The first step 4-{[(2S,4S)-N-tert-butoxycarbonyl-4-(3-hydroxy-2,2-dimethylpropylamino)-pyrrol-2-yl]-formyl}thio Preparation of Morpholine 2b
[0136] 4-{[(2S,4S)-4-Amino-N-tert-butoxycarbonyl-pyrrol-2-yl]-formyl}thiomorpholine 6a (0.15g, 0.48mmol) was mixed with 2,2-bis Methyl-3-hydroxypropanal (0.051g, 0.50mmol) was dissolved in 10mL of dichloromethane, after complete dissolution, a small amount of acetic acid was added to make the reaction system pH=3-5, stirred at room temperature for 3 hours, and the reaction system was cooled to 0°C Next, sodium triacetylborohydride (0.317 g, 1.50 mmol) was added, raised to room temperature and stirred for 6 hours until the reaction was complete. Add 20 mL of water to the reaction solution, extract with dichloromethane and combine the organic layers, wash with saturated sodium bicarbonat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com