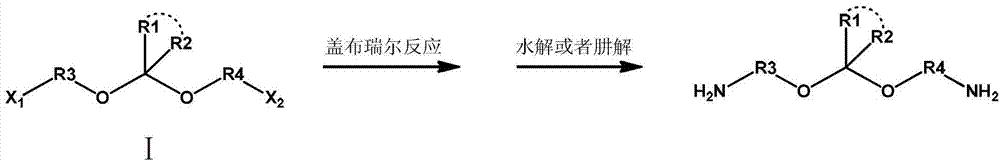
A kind of preparation method of degradable primary amine curing agent
A curing agent and primary amine technology, applied in the field of preparation of degradable primary amine curing agent, can solve the problems of strong acid corrosion, fiber shortening, difficult post-processing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] Embodiment 1: the synthesis of two (2-chloroethoxy) methane
[0142]
[0143] Method 1: Put 346 grams of toluene and 800 grams of 2-chloroethanol into the reaction flask in sequence, and put 164 grams of paraformaldehyde and 4.8 grams of p-toluenesulfonic acid under stirring, and the system is heated up slowly, and the Dean-Stark is refluxed to separate water. When no water droplets were separated, the reaction was extended for another 1 hour, which was the end of the reaction. After the reaction, lower the temperature to below 40°C, add an appropriate amount of sodium carbonate to the reaction bottle, adjust the pH value of the reaction liquid system to be close to neutral, then concentrate the reaction liquid under reduced pressure, recover all the toluene, vacuum distill the residue, and collect 60-75 °C / 200pa, about 753 grams of bis(2-chloroethoxy)methane was obtained, and the yield was 87.6%.
[0144] Method 2: Put 346 grams of toluene and 800 grams of 2-chloro...
Embodiment 2
[0145] Embodiment 2: the synthesis of 2,2-bis(2-chloroethoxy)propane
[0146]
[0147] 20 grams of 2-chloroethanol, 14.2 grams of 2,2-dimethoxypropane, 0.11 grams of p-toluenesulfonic acid, and 0.35 liters of toluene were mixed at room temperature, DEAN-STARK was refluxed to separate water, and reacted for 20 hours. Concentrate under reduced pressure at room temperature to recover toluene, and collect the residue with a boiling range of 53-55°C under vacuum at 150 Pa to obtain 13 g of 2,2-bis(2-chloroethoxy)propane.
Embodiment 3
[0148] Embodiment 3: the synthesis of curing agent 1
[0149]
[0150] Curing agent I
[0151] Curing agent I put 960 grams of concentrated ammonia water and 150 grams of ammonium chloride into the autoclave, stir and dissolve, then add 5 grams of bis(2-chloroethoxy)methane prepared in Example 1, then heat up to 120 ° C, Stir the reaction for 3 hours, and check the end point of the reaction by TLC. After the reaction, concentrate most of the solution under reduced pressure, transfer the residual solution to a reaction bottle, and adjust the pH to ≥ 10 with solid sodium hydroxide solution at a temperature below 25°C, and then use chloroform to and ethanol (volume ratio 3:1) mixed solvent 300 ml, extract the aqueous phase 3 times, combine the organic phase, dry the organic phase with anhydrous sodium sulfate, filter, wash the filter cake once with a small amount of solvent, and concentrate the filtrate to dryness , Distilled under reduced pressure to collect under 70Pa vacuu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com