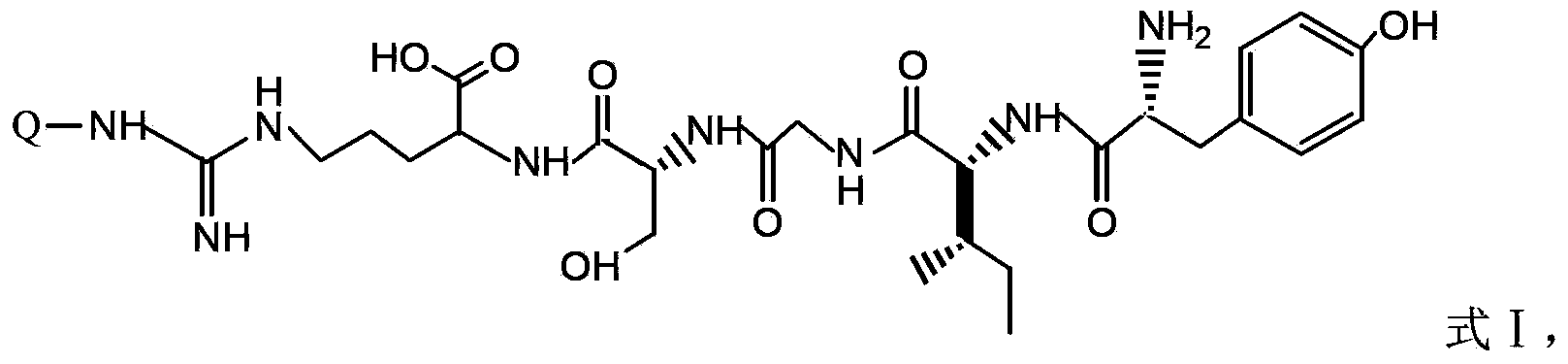
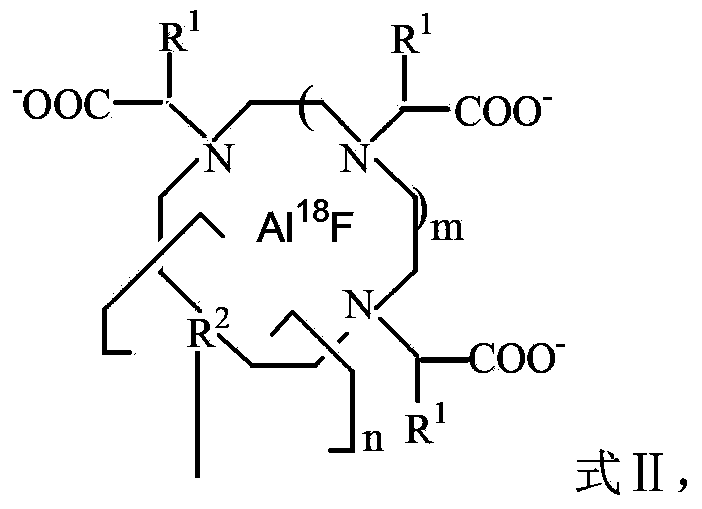
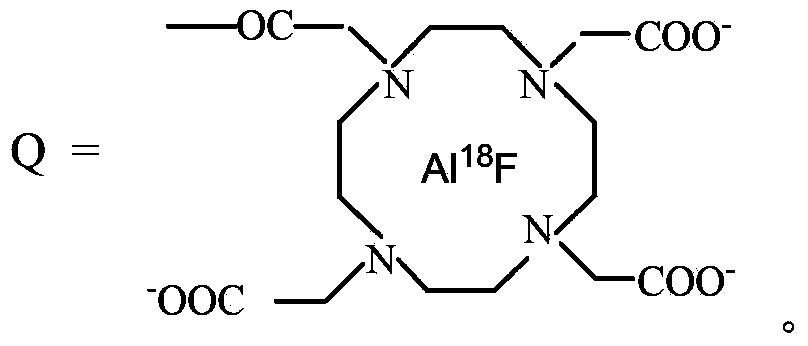
18F-fluorine labeling pentapeptide complex and synthetic method thereof
A synthesis method and fluorine labeling technology, applied in the fields of medicine and chemistry, can solve the problems of short retention time, less research, low utilization rate, etc., and achieve the effects of good thermal stability, good water solubility and small dosage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 0.4g of 1,4,7-triazacyclonona-1,4,7-triacetic acid mono-N-hydroxysuccinimide active ester (1mmol) was added to the pentapeptide compound YIGSR (0.592g, 1mmol) and dissolved in 20mL N,N-dimethylformamide solution, electromagnetically stirred at room temperature for 6 hours. After filtration, the filtrate was evaporated under reduced pressure to remove the solvent, and dried in vacuo to obtain 0.83 g of the ligand YIGSR-NOTA compound with a yield of 84%.
[0043] eluted with carbonate 18 F-fluoride solution (5.39GBq) was dissolved in 50 μL 0.4mol / mL K 2 CO 3 , neutralized with 5 μL glacial acetic acid, and added 1 μL 0.01mol / mL AlCl 3 ·6H 2 O was dissolved in 0.1mol / mL NaOAc solution (pH4), followed by adding 2μL of 0.01mol / mL ligand YIGSR-NOTA compound (0.01754g, 20nmol) dissolved in 0.1mol / mL NaOAc solution (pH4), at 105℃ Reaction 17min. Cool, with 1mL H 2 O dilutes the reaction mixture solution, separates and purifies with column chromatography, obtains 18 F-fluo...
Embodiment 2
[0045] 0.46g 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid mono-tert-butylene active ester (1mmol) was added to the pentapeptide compound YIGSR (0.592g, 1mmol) In a solution of 30 mL of dimethyl sulfoxide, electromagnetically stirred at room temperature for 4 hours. After filtration, the filtrate was evaporated under reduced pressure to remove the solvent, and dried in vacuo to obtain 0.789 g of the ligand DOTA-YIGSR compound with a yield of 75%.
[0046] eluted with carbonate 18F - Fluoride solution (5.39GBq) was dissolved in 50 μL 0.4mol / mL K 2 CO 3 , neutralized with 5 μL glacial acetic acid, and added 1 μL 0.01mol / mL AlCl 3 ·6H 2 O was dissolved in a solution (pH4) of 0.1mol / mL NaOAc, followed by adding 2 μL of 0.01mol / mL ligand DOTA-YIGSR compound compound (0.01956g, 20nmol) was dissolved in a solution (pH4) of 0.1mol / mL NaOAc, in React at 105°C for 17 minutes. Cool, with 1mL H 2 O dilutes the reaction mixture solution, separates and purifies with column...
Embodiment 3
[0048] 0.486g of 1,4,7-triazacyclonona-1,4,7-triacetic acid-2-p-benzyl bromide (1mmol) was added to the pentapeptide compound YIGSR (0.592g, 1mmol) dissolved in 20mL N,N - To the solution of dimethylformamide, triethylamine (0.101 g, 1 mmol) was added, and electromagnetically stirred at room temperature for 12 hours. Filtration, the filtrate was evaporated under reduced pressure to remove the solvent, and dried in vacuo to obtain 0.89g ligand NOTA-C 6 h 4 CH 2 - YIGSR compound, yield 90%.
[0049] washed with salt 18 Dissolve F-fluoride solution (70.3MBq) in 50 μL of normal saline, neutralize with 5 μL of glacial acetic acid, add 2 μL of 0.01mol / mL AlCl 3 ·6H 2 O was dissolved in a 0.1mol / mL NaOAc solution (pH4), followed by the addition of 20 μL of the ligand NOTA-C 6 h 4 CH 2 -YIGSR compound (0.03932g, 2mmol / mL, 40nmol) was dissolved in 0.1mol / mL NaOAc solution (pH4), and reacted at 105°C for 15min. Cool, dilute the reaction mixture solution with 1mL PBS (pH7.4), se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com