Preparation method for GAP base elastomer with non-isocyanate curing manner
A non-isocyanate, curing technology, applied in the field of preparation of GAP-based elastomers, can solve the problems of mechanical properties of materials and adverse effects of application effects, and achieve the effects of good mechanical properties, less interference, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A kind of preparation method of the GAP-based elastomer of non-isocyanate curing mode, it comprises the steps:
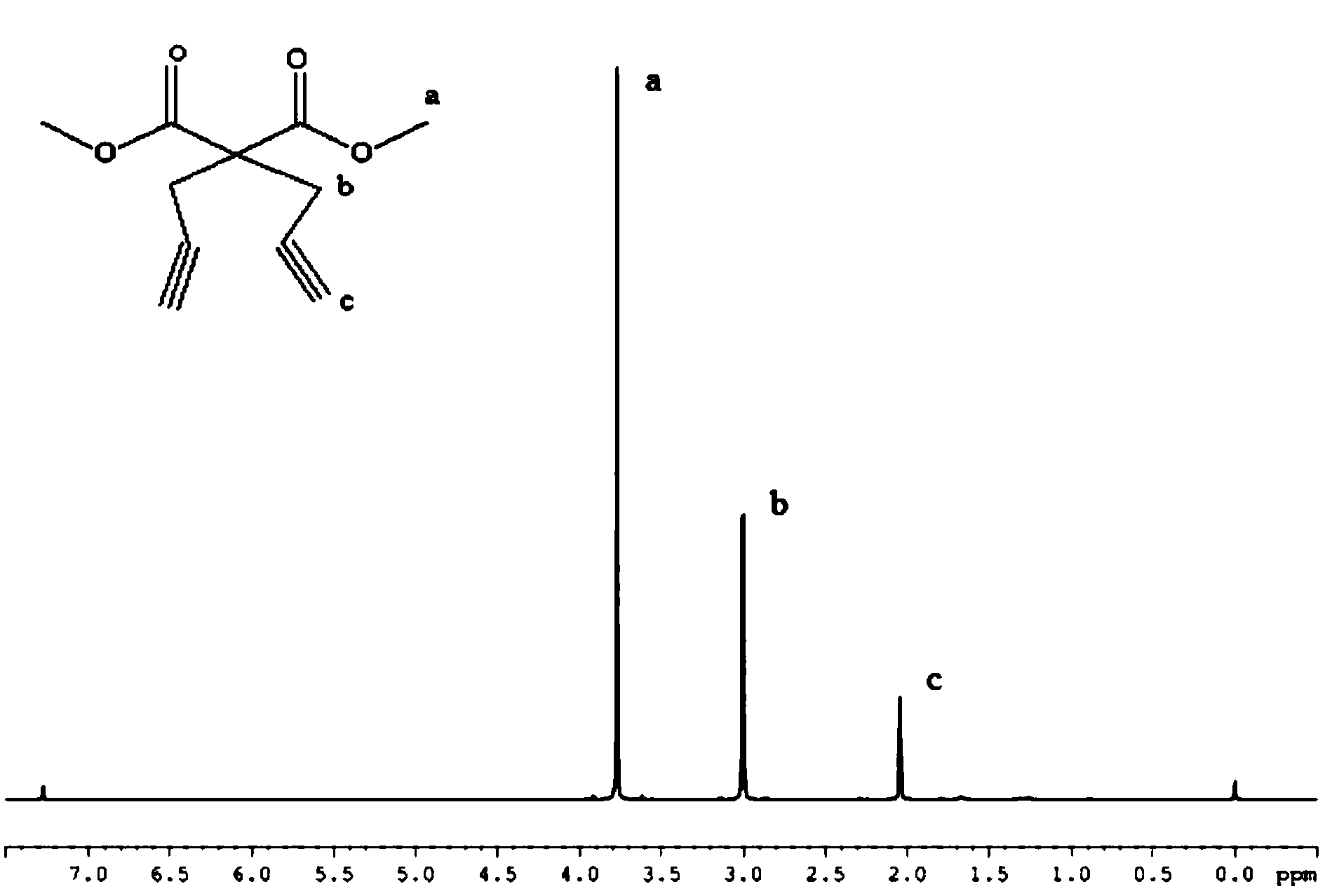
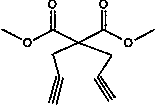
[0032] 1) Preparation of dimethyl 2,2-propargylmalonate:
[0033] a. Weigh 3.36g of 70wt% NaH and dissolve in 40mL of anhydrous THF to obtain a THF solution containing NaH; another 4.86ml of dimethyl malonate is dissolved in 40mL of anhydrous THF to obtain a dimethyl malonate solution ; 10.1ml propargyl bromide is added dropwise in the 80ml anhydrous tetrahydrofuran solution, obtains the solution of propargyl bromide;
[0034] b. Slowly add the dimethyl malonate solution into the THF solution of NaH at room temperature, and continue stirring for 1 h to obtain the reaction solution A; slowly transfer the propargyl bromide solution to the reaction solution A [Dimethyl malonate : Sodium hydride: the mol ratio of propargyl bromide=1:2.3:2.5], at 110 ℃, reflux reaction 3h, obtain reaction solution C;
[0035] Add (3×50mL) saturated ammonium chloride solution t...
Embodiment 2
[0043]A kind of preparation method of the GAP-based elastomer of non-isocyanate curing mode, it comprises the steps:
[0044] 1) Preparation of dimethyl 2,2-propargylmalonate:
[0045] a. Weigh 3.36g of 70wt% NaH and dissolve in 40mL of anhydrous THF to obtain a THF solution containing NaH; another 4.86ml of dimethyl malonate is dissolved in 40mL of anhydrous THF to obtain a dimethyl malonate solution ; 10.1ml propargyl bromide is added dropwise in the 80ml anhydrous tetrahydrofuran solution, obtains the solution of propargyl bromide;
[0046] b. Slowly add the dimethyl malonate solution into the THF solution of NaH at room temperature, and continue stirring for 1 h to obtain the reaction solution A; slowly transfer the propargyl bromide solution to the reaction solution A [Dimethyl malonate : Sodium hydride: the mol ratio of propargyl bromide=1:2.3:2.5], at 110 ℃, reflux reaction 3h, obtain reaction solution C;
[0047] Add (3×50mL) saturated ammonium chloride solution to...
Embodiment 3
[0053] A kind of preparation method of the GAP-based elastomer of non-isocyanate curing mode, it comprises the steps:
[0054] a. Weigh 3.36g of 70wt% NaH and dissolve in 40mL of anhydrous THF to obtain a THF solution containing NaH; another 4.86ml of dimethyl malonate is dissolved in 40mL of anhydrous THF to obtain a dimethyl malonate solution ; 10.1ml propargyl bromide is added dropwise in the 80ml anhydrous tetrahydrofuran solution, obtains the solution of propargyl bromide;
[0055] b. Slowly add the dimethyl malonate solution into the THF solution of NaH at room temperature, and continue stirring for 1 h to obtain the reaction solution A; slowly transfer the propargyl bromide solution to the reaction solution A [Dimethyl malonate : Sodium hydride: the mol ratio of propargyl bromide=1:2.3:2.5], at 110 ℃, reflux reaction 3h, obtain reaction solution C;
[0056] Add (3×50mL) saturated ammonium chloride solution to the reaction solution C and extract 3 times, separate the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Elastic modulus | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com