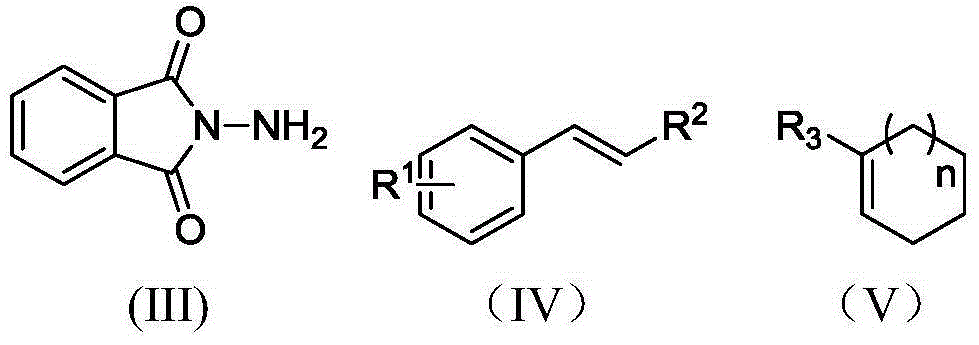
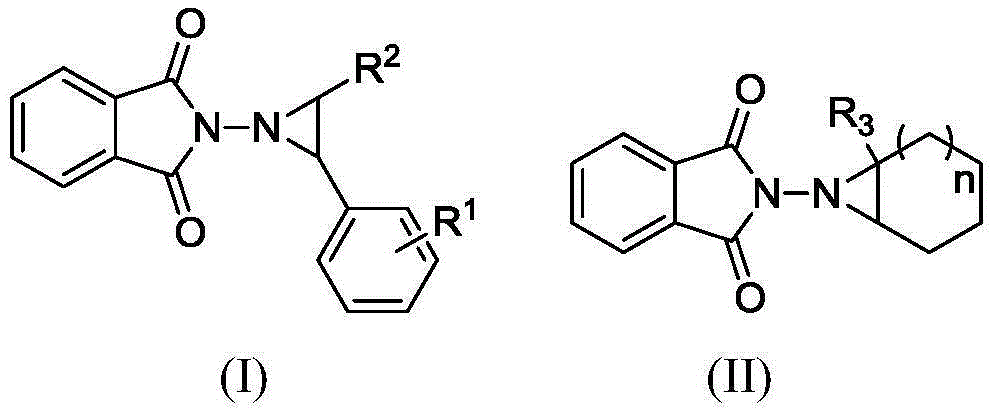
Electrochemical catalytic synthesis method of aziridine compounds
An aziridine and a synthesis method technology are applied in the field of electrochemical catalysis synthesis of aziridine compounds, can solve the problems of complex electrolysis device, high decomposition voltage, high price, etc. Mild reaction conditions and low decomposition voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the electrochemical catalysis aziridine of styrene
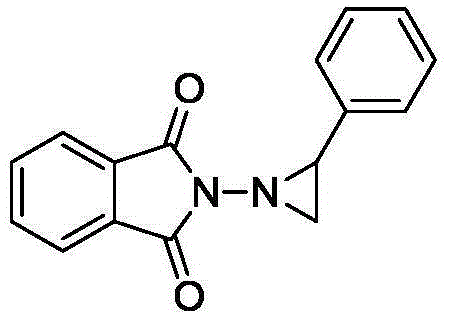
[0029] In a 50 mL single-chamber electrolytic cell, N-aminophthalimide (1.3 mmol), styrene (1 mmol), potassium carbonate (1 mmol) and tetrabutylammonium iodide (0.2 mmol) were added to the solution In 15mL of 2,2,2-trifluoroethanol solution with 0.1M triethylamine / acetic acid, with glassy carbon electrode as anode and iron sheet as cathode, at 4mA / cm 2 Electrolyze at a constant current, stir at room temperature, stop electrolysis when the energized amount reaches 3F / mol, remove the solvent, dissolve with dichloromethane, wash with water three times, and separate by column chromatography to obtain N-(aminophthaloyl imine)-2-phenylaziridine. Yield: 50%.
[0030]
[0031] yellowsolid;149.4-150.3℃; 1 HNMR (400MHz, CDCl3): δ=7.83-7.80(m,2H),7.73-7.71(m,2H),7.37(d,J=8Hz,2H),7.22(d,J=8Hz,2H),3.59 (dd, J=8.0,6.0Hz,1H),2.89(dd,J=8,2.4Hz,1H),2.81(dd,J=6,2.4Hz,1H).
Embodiment 2
[0032] Example 2: Electrochemically catalyzed aziridine of styrene
[0033] In a 50 mL single-chamber electrolytic cell, N-aminophthalimide (1.3 mmol), styrene (1 mmol), potassium carbonate (1 mmol) and tetrabutylammonium iodide (0.2 mmol) were added to the solution In 15mL2,2,2-trifluoroethanol solution with 0.05M lithium perchlorate, with glassy carbon electrode as anode and iron sheet as cathode, at 4mA / cm 2 Electrolyze at a constant current, stir at room temperature, stop electrolysis when the energized amount reaches 3F / mol, remove the solvent, dissolve with dichloromethane, wash with water three times, and separate by column chromatography to obtain N-(aminophthaloyl imine)-2-phenylaziridine. Yield: 67%.
Embodiment 3
[0034] Example 3: Electrochemically catalyzed aziridine of styrene
[0035] In a 50 mL single-chamber electrolytic cell, N-aminophthalimide (1.3 mmol), styrene (1 mmol), potassium carbonate (1 mmol) and tetraethylammonium iodide (0.2 mmol) were added to the solution In 15mL2,2,2-trifluoroethanol solution with 0.05M lithium perchlorate, with glassy carbon electrode as anode and iron sheet as cathode, at 4mA / cm 2 Electrolyze at a constant current, stir at room temperature, stop electrolysis when the energized amount reaches 3F / mol, remove the solvent, dissolve with dichloromethane, wash with water three times, and separate by column chromatography to obtain N-(aminophthaloyl imine)-2-phenylaziridine. Yield: 51%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com