Rapidly released fexofenadine pills and preparation method thereof
A fexofenadine and fast technology, applied in the field of medicine, can solve the problems of long operation process, difficult and rapid release, danger, etc., and achieve the effects of shortening the drug application time, improving production efficiency and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
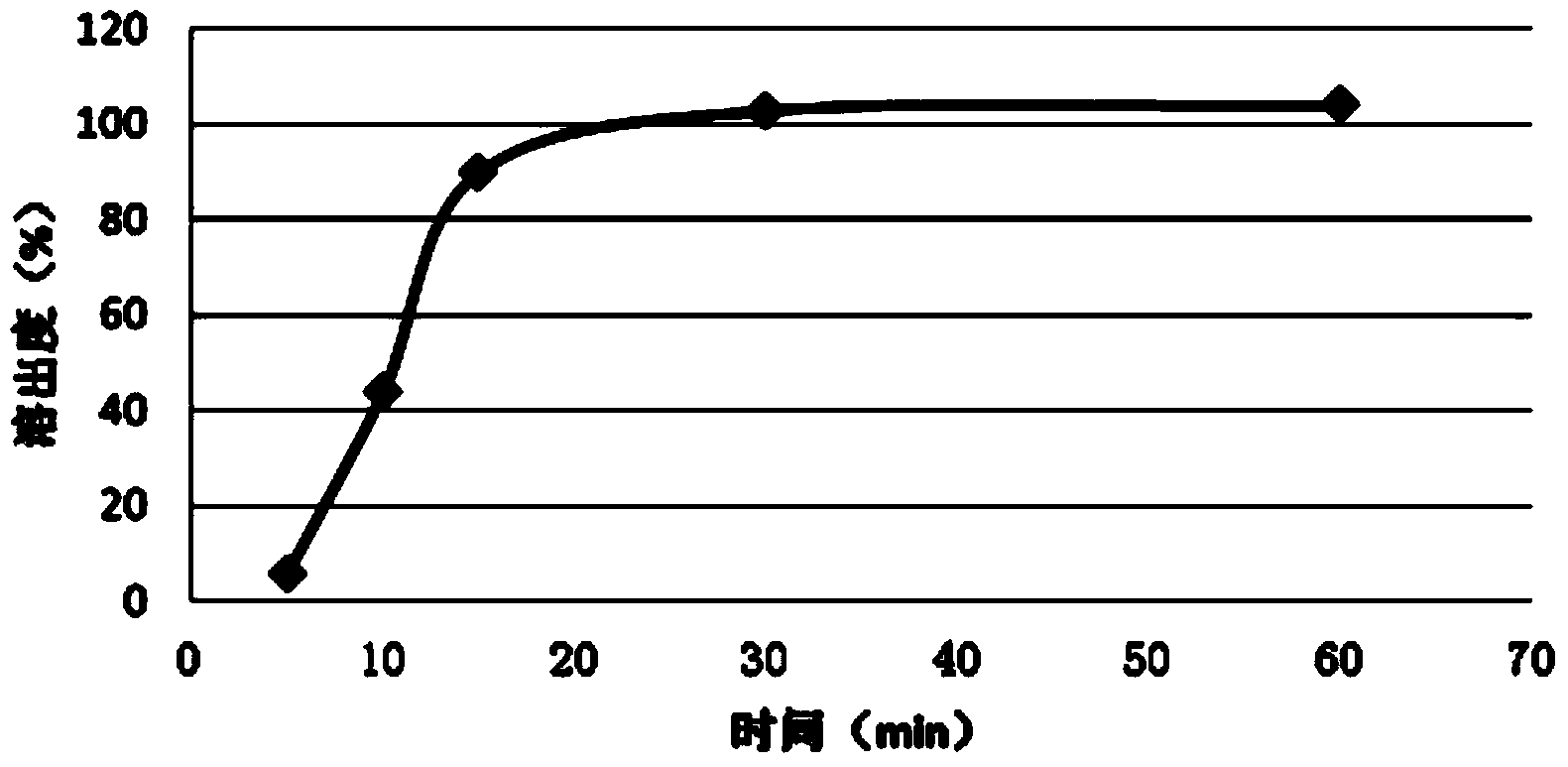
Embodiment 1
[0030] A kind of fast-release fexofenadine pellet provided by the present invention is made by wrapping the fexofenadine suspension on the surface of the pseudoephedrine sustained-release pellet, and the fexofenadine suspension prescription is: fexofenadine Set 100g, hypromellose 8g, pregelatinized starch 20g, talcum powder 10g, water 500g.
[0031] Its preparation method comprises the following steps in turn:
[0032] 1) Preparation of fexofenadine suspension
[0033] Weigh 500g of purified water, add 8g of hypromellose and 20g of pregelatinized starch under stirring, stir (800rpm) evenly and then slowly add fexofenadine, when the suspension starts to thicken, stop adding For fexofenadine, add an appropriate amount of talcum powder, keep stirring (800rpm) until the suspension becomes thinner, then slowly add fexofenadine, stop fexofenadine when the suspension starts to thicken, Add the right amount of talcum powder, keep stirring (800rpm) until the suspension becomes thinne...
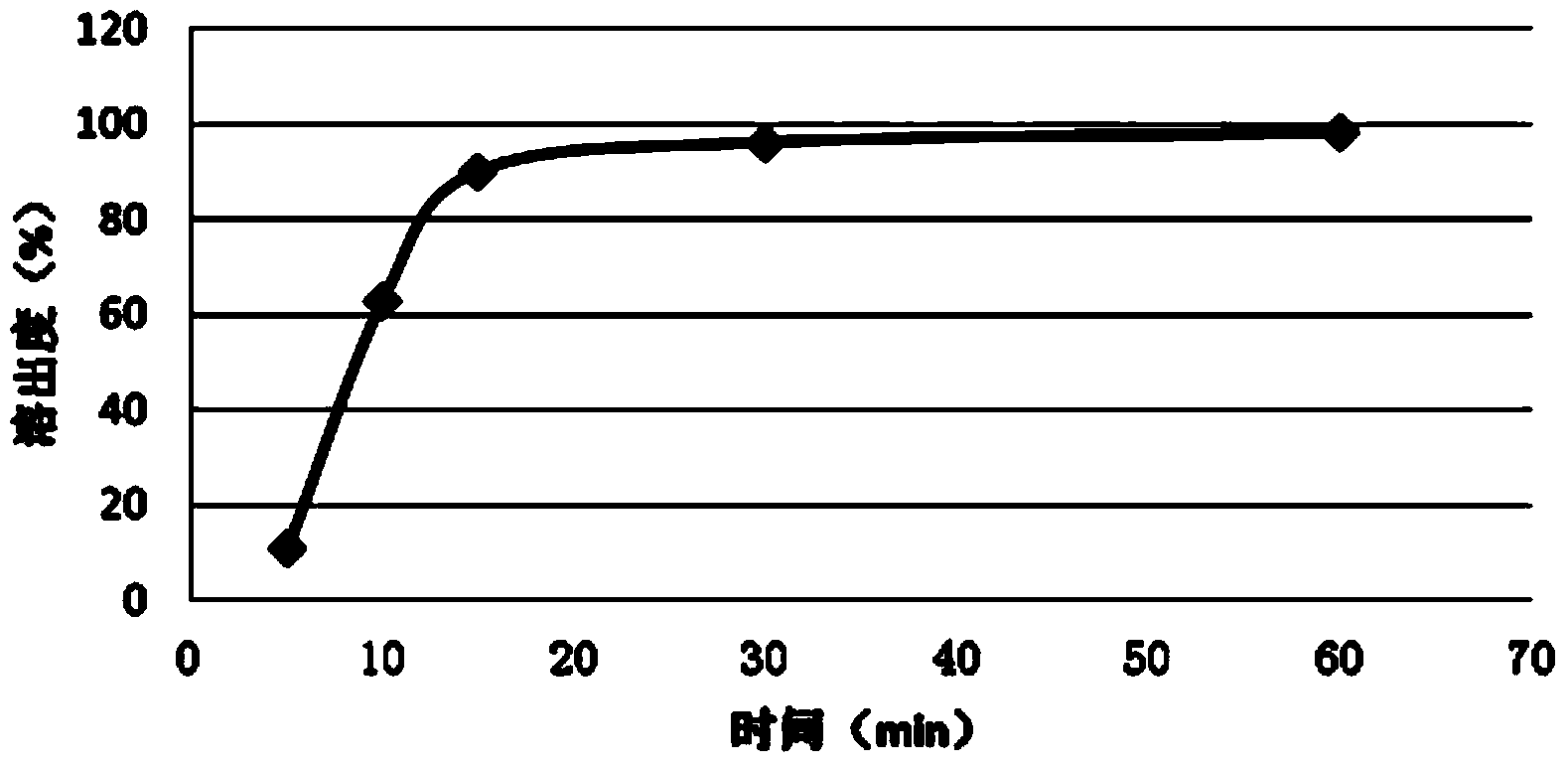
Embodiment 2
[0038] A kind of fast release fexofenadine pellet provided by the present invention is formed by wrapping the fexofenadine suspension on the surface of the pseudoephedrine sustained release pellet, and the fexofenadine suspension prescription is: fexofenadine 60g, hydroxypropyl cellulose 3g, sodium carboxymethyl starch 6g, magnesium stearate 2g, water 409g.
[0039]The preparation method comprises the steps in turn:
[0040] 1) Preparation of fexofenadine suspension
[0041] Weigh 409g of purified water, add 3g of hydroxypropyl cellulose and 6g of sodium carboxymethyl starch under stirring, stir (1200rpm) evenly and then slowly add fexofenadine, when the suspension starts to thicken, stop adding For fexofenadine, add an appropriate amount of magnesium stearate, keep stirring (1200rpm) until the suspension becomes thinner, then slowly add fexofenadine, stop fexofenadine when the suspension starts to thicken Set, add an appropriate amount of magnesium stearate, continue to sti...
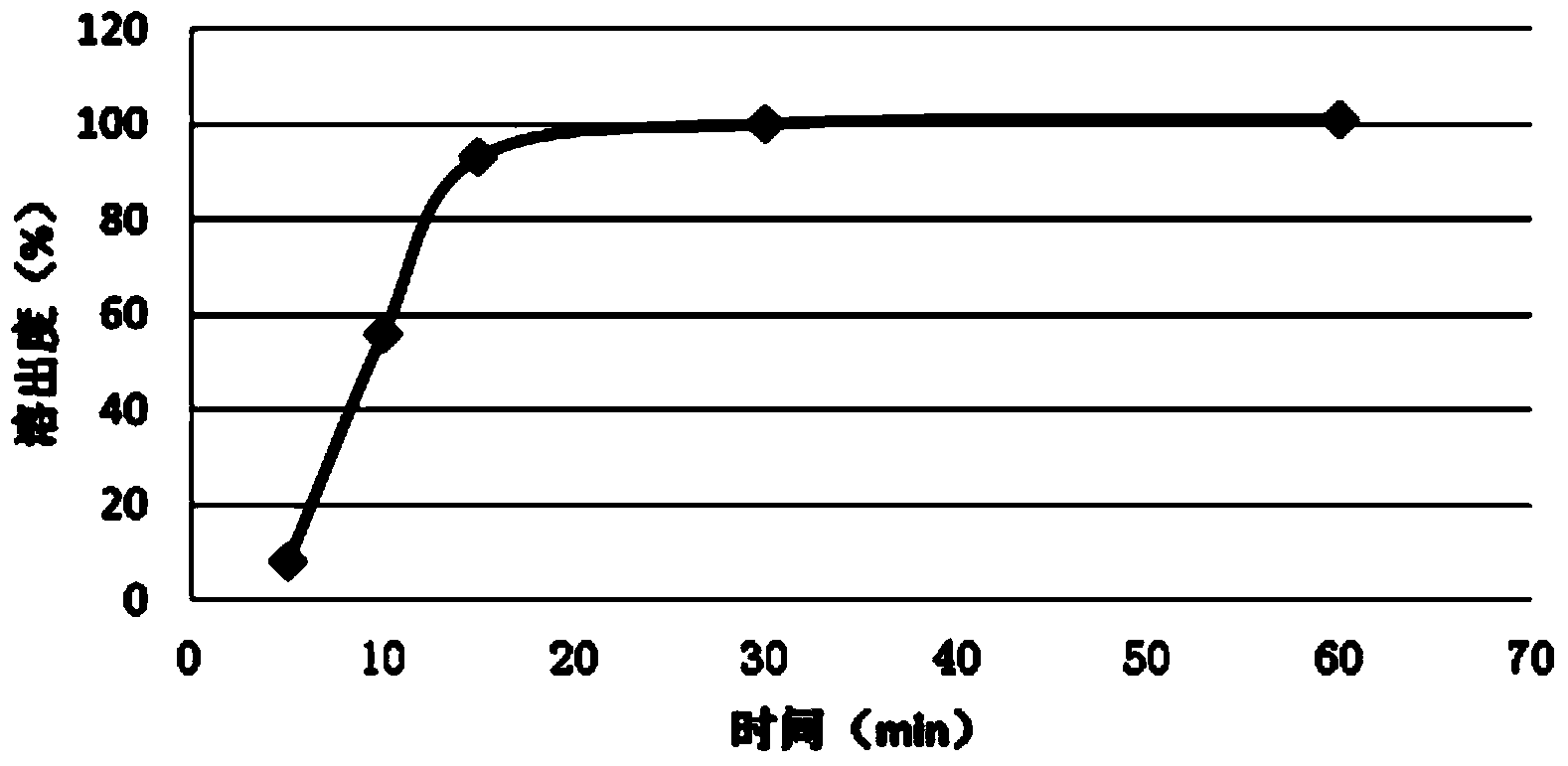
Embodiment 3
[0046] Another fast-release fexofenadine pellet provided by the present invention is formed by wrapping the fexofenadine suspension on the surface of the pseudoephedrine sustained-release pellet, and the fexofenadine suspension prescription is: fexofenadine Calcium 120g, copovidone 6g, microcrystalline cellulose 10g, calcium stearate 4g, water 360g.
[0047] The preparation method comprises the steps in turn:
[0048] 1) Preparation of fexofenadine suspension
[0049] Weigh 360g of purified water, add 6g of copovidone and 10g of microcrystalline cellulose under stirring, stir (1500rpm) evenly and slowly add fexofenadine, stop adding fexofenadine when the suspension starts to thicken For fenadine, add an appropriate amount of calcium stearate, keep stirring (1500rpm) until the suspension becomes thinner, then slowly add fexofenadine, and stop fexofenadine when the suspension begins to thicken, Add an appropriate amount of calcium stearate, keep stirring (1500rpm) until the su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com