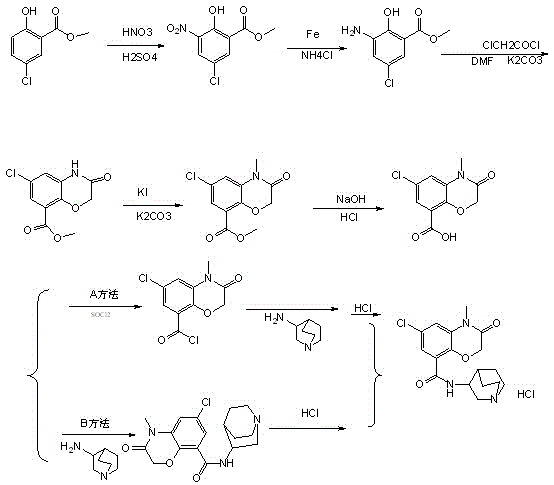
A kind of synthetic method of azasetron hydrochloride
The technology of azasetron hydrochloride and synthesis method is applied in the field of medicine, which can solve the problems of heavy sulfur oxychloride environmental pollution, long route, difficult operation, unsuitable for scale-up production, etc., and achieve easy operation, short synthesis route and high yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
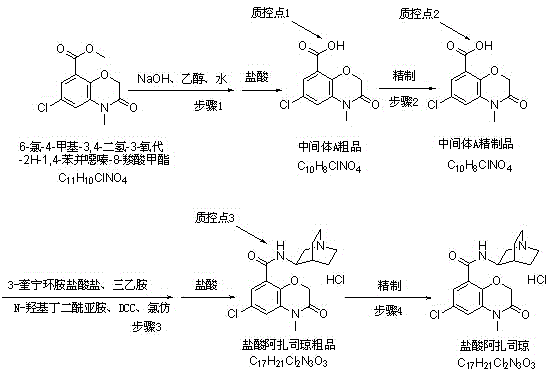
[0034] (1) Preparation of azasetron hydrochloride intermediate (C 10 h 8 ClNO 4 )Crude
[0035] S1. Prepare dilute hydrochloric acid by taking concentrated hydrochloric acid and purified water at a weight ratio of 1:0.9;
[0036] S2. Add purified water and sodium hydroxide to the reaction kettle, wherein the weight ratio of sodium hydroxide to purified water is 1:19, and stir until the sodium hydroxide is completely dissolved, then add absolute ethanol, and cool down to 25~ 30°C, finally add 6-chloro-4-methyl-3,4-dihydro-3-oxo-2H-1,4-benzoxazine-8-carboxylic acid methyl ester, keep stirring, and track with TLC After the reaction until the raw materials are completely reacted, the reaction solution A is prepared for future use, wherein, 6-chloro-4-methyl-3,4-dihydro-3-oxo-2H-1,4-benzoxazine- The mass of methyl 8-carboxylate: the mass of sodium hydroxide: the volume of absolute ethanol is 1:0.45:3.8, the mass is in g, and the volume is in ml;
[0037] S3. Add the reaction s...
Embodiment 2
[0057] The steps of this embodiment are consistent with Embodiment 1, the difference is:
[0058] The weight ratio of concentrated hydrochloric acid and purified water in S1 is 1:1;
[0059] In S2, the weight ratio of sodium hydroxide to purified water is 1:20, 6-chloro-4-methyl-3,4-dihydro-3-oxo-2H-1,4-benzoxazine-8 - the mass of methyl carboxylate: the mass of sodium hydroxide: the volume of absolute ethanol is 1: 0.47: 3.9, the mass is in g and the volume is in ml;
[0060] Dilute hydrochloric acid 6-chloro-4-methyl-3,4-dihydro-3-oxo-2H-1,4-benzoxazine-8-carboxylic acid methyl ester and dilute hydrochloric acid in reaction solution A in S3 The mass ratio is 1:4.6;
[0061] The mass ratio of azasetron hydrochloride intermediate crude product, absolute ethanol and purified water in S5 is 1:21:3;
[0062] The mass ratio of each component in S7 is--Azasetron hydrochloride intermediate refined product: 3-aminoquinuclidine hydrochloride: N-hydroxysuccinimide: triethylamine: N,...
Embodiment 3
[0073] The steps of this embodiment are consistent with Embodiment 1, the difference is:
[0074] The weight ratio of concentrated hydrochloric acid to purified water in S1 is 1:1.2;
[0075] The weight ratio of sodium hydroxide to purified water in S2 is 1:21,6-chloro-4-methyl-3,4-dihydro-3-oxo-2H-1,4-benzoxazine-8- The mass of methyl carboxylate: the mass of sodium hydroxide: the volume of absolute ethanol is 1:0.5:4, the mass is in g, and the volume is in ml;
[0076] The mass ratio of dilute hydrochloric acid 6-chloro-4-methyl-3,4-dihydro-3-oxo-2H-1,4-benzoxazine-8-carboxylic acid methyl ester to dilute hydrochloric acid in S3 is 1 :5;
[0077] The mass ratio of azasetron hydrochloride intermediate crude product, absolute ethanol and purified water in S5 is 1:2:4;
[0078] The mass ratio of each component in S7 is azasetron hydrochloride intermediate refined product: 3-aminoquinuclidine hydrochloride: N-hydroxysuccinimide: triethylamine: N,N'-dicyclic Hexylcarbodiimide...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com