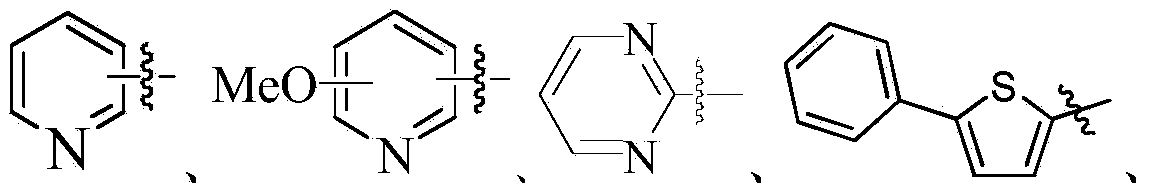
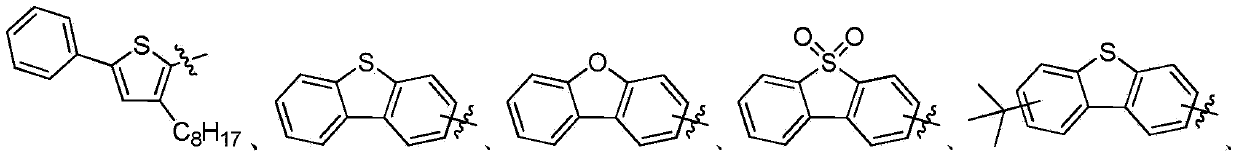
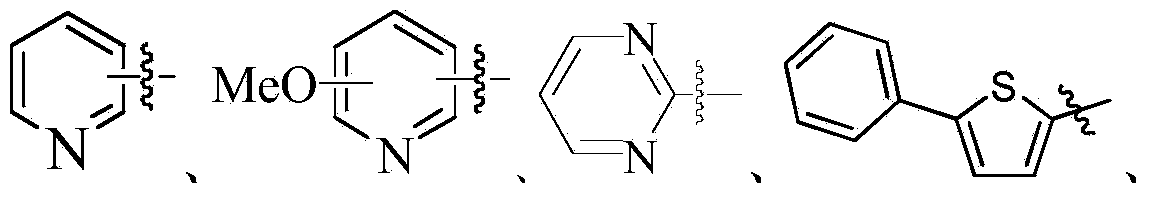
OLED material with carrier transmittability as well as preparation method and application thereof
A technology of transport ability and carrier, which is applied in the preparation of organic compounds, luminescent materials, and preparation of carboxylic acid nitriles, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144] Preparation of compound LM005:
[0145] 1.1 Preparation of Intermediate S-1:
[0146]
[0147] Add 0.1mol titanium trichloride-aluminum chloride (78.5%) and 200ml anhydrous THF to the reaction flask, add 0.05mol lithium aluminum hydride under stirring at room temperature, and stir for 10 minutes; then slowly drop 0.049mol triethylamine into In the reaction flask, and stirred for 1 hour; then 10mmol of raw material 5-dibenzocycloheptenone (S-0) and 10mmol of 2,7-dibromo-9-fluorenone were added to the reaction flask, heated to reflux and stirred React for 20 hours; after the reflux reaction is completed, cool to room temperature, slowly drop 200ml of water into the reaction flask, and suction filter after stirring; The residue obtained after drying was recrystallized from ethanol to obtain 3.84 g of a yellow solid, namely intermediate S-1; the yield was 75%.
[0148] 1.2 Preparation of compound LM005:
[0149]
[0150] Add 4mmol of intermediate S-1, 8.6mmol of ...
Embodiment 2
[0157] Preparation of compound LM052:
[0158] 2.1 Preparation of intermediate S-1:
[0159] Intermediate S-1 was prepared with reference to step 1.1 in Example 1, the difference being that the raw material 5-dibenzocycloheptenone in step 1.1 of Example 1 was replaced by 3,7-difluoro-5-dibenzo Cycloheptenone, its consumption is constant.
[0160] 2.2 Preparation of compound LM052:
[0161]
[0162] Add 3.6mmol of intermediate S-1, 9mmol of 4-pyridineboronic acid, 21.7mmol of anhydrous potassium carbonate, 50mg of Pd(PPh 3 ) 4 Catalyst, 100ml of toluene, 50ml of ethanol and 50ml of water, under the protection of nitrogen, heat up and reflux for 24 hours; after the reaction, cool to room temperature; The organic phases were combined; the organic phase was concentrated to dryness under reduced pressure, and the obtained residue was separated and purified with a silica gel column to finally obtain 1.4 g of a yellow solid, namely compound LM052; the yield was 71%.
[0163...
Embodiment 3
[0169] Preparation of compound LM006:
[0170] 3.1 Preparation of Intermediate S-1:
[0171] Intermediate S-1 was prepared referring to step 1.1 in Example 1.
[0172] 3.2 Preparation of compound LM052:
[0173]
[0174] Add 4mmol of intermediate S-1, 9.6mmol of carbazole, 11.4mmol of sodium tert-butoxide and 100ml of xylene into the reaction flask, and then add 25mg of Pd(OAc) under stirring 2 Catalyst and 45mg of tert-butylphosphine, under the condition of nitrogen protection, reflux reaction for 10-12 hours; after the reaction, cool to room temperature, add 50ml of water to the reaction bottle, stir and separate the layers, separate the organic phase, and use the water phase Extract with dichloromethane; collect and combine organic phases, add anhydrous MgSO 4 Drying and suction filtration; the filtrate was concentrated to dryness under reduced pressure, and the obtained residue was separated and purified with a silica gel column; the obtained product was recrystall...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com