Corn phenylalanine ammonia enzyme and application thereof
A technology of phenylalanine ammonia lyase and corn, which is applied in the fields of genetic engineering and microorganisms, can solve problems such as the research on the in vitro expression of corn phenylalanine ammonia lyase gene that has not been found, and achieves guaranteed safety and high application value Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Construction of recombinant bacteria containing maize phenylalanine ammonia lyase gene sequence.
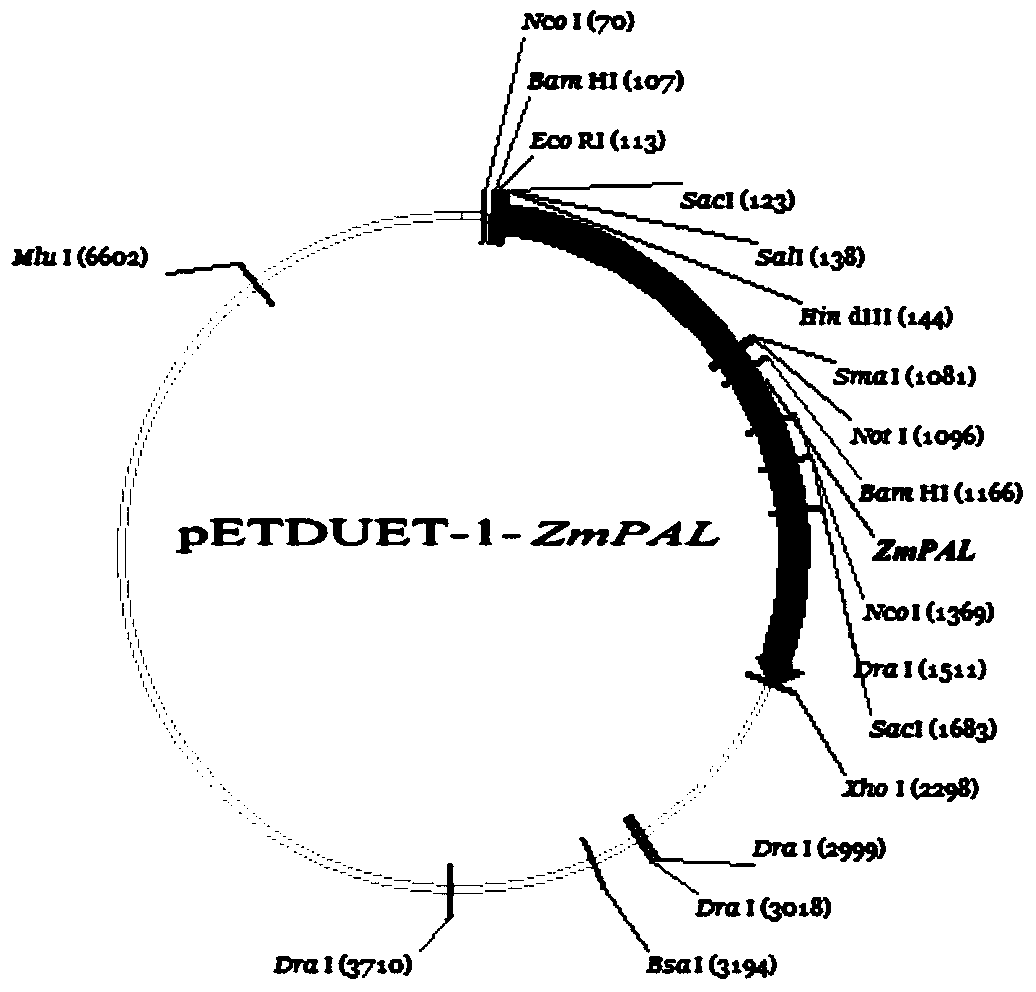
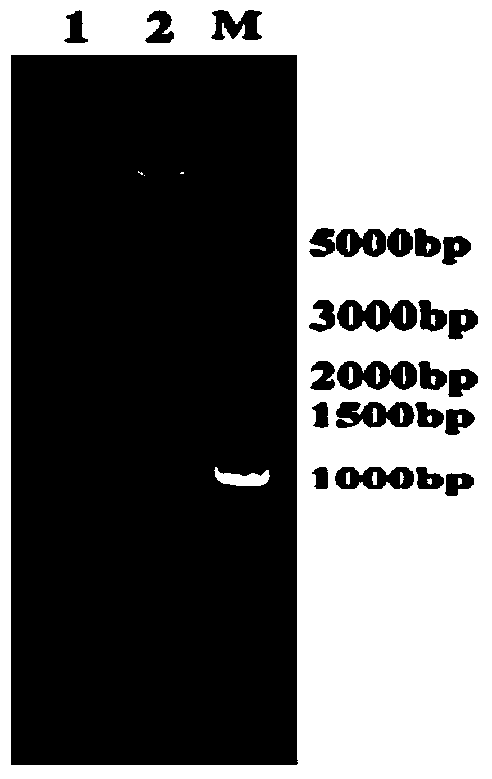
[0027] Synthesize the phenylalanine ammonia-lyase gene (shown in SEQ ID No: 1) fragment encoding maize in the present invention by chemical synthesis, connect it to the pETDuet-1 vector, transform it into Escherichia coli competent cells, and undergo Blue and white spot screening, extraction of positive cloned plasmids and enzyme digestion map analysis, verification of the recombinant plasmid pETDuet-1-ZmPAL carrying the ZmPAL gene, and the recombinant expression vector pETDuet-1-ZmPAL as shown in the figure figure 1 shown. The recombinant vector recombinant plasmid pETDuet-1-ZmPAL was transformed into Escherichia coli BL21 (DE3) competent cells, the transformed cells were spread on LB plates containing 1‰Amp and cultured at 37°C, and the single cells grown on the plates were Colony extraction plasmid was verified by double restriction electrophoresis, and the ...
Embodiment 2
[0028] Example 2: Prokaryotic expression and purification of maize phenylalanine ammonia lyase gene.
[0029] The clones identified correctly in Example 1 were cultured overnight, and then transferred to LB medium containing 1 μg / mL ampicillin for culture, until the bacterial solution OD 600 When the concentration is 0.6-0.8, add IPTG with a final concentration of 1mM to induce expression at 16°C. After induction for 8 hours, collect the bacterial liquid, 8000rpm, 5min, pour off the supernatant medium, wash twice with PBS equal volume, add an appropriate amount of PBS, making the resuspension solution OD 600=20, add 15% (v / v) glycerin, 1mM phenylmethylsulfonyl fluoride (PMSF), and mix on a vortex machine; use an ultrasonic crusher to crush, ultrasonic crushing conditions: the working time is set to 20min, every Work for 5 seconds, rest for 4 seconds, set the power to 25%, obtain the crude enzyme solution of phenylalanine ammonia lyase after crushing, centrifuge at 10,000 rpm ...
Embodiment 3
[0030] Example 3: Research on the enzymatic properties of phenylalanine ammonia lyase.
[0031] The crude enzyme solution and pure enzyme solution obtained in Example 2 were studied on enzymatic properties, including enzyme activity, specific activity, optimum temperature and optimum pH, etc. Enzyme activity was measured by UV spectrophotometer.
[0032] For the determination of phenylalanine ammonia-lyase enzyme activity, add each component according to the reaction system shown in Table 1, and use the inactivated enzyme solution as the control tube, incubate at 37°C, react for 30min, and then stop the reaction with 6N HCl respectively. A UV spectrophotometer was used to 290 Measure the amount of cinnamic acid produced before and after the reaction, and use the difference between the two to calculate the enzyme activity of the cell crude enzyme solution PAL (the unit of enzyme activity is the international unit IU, that is, 1 μmol of cinnamic acid produced per minute is 1 IU...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com