Method for synthesizing doxycycline hydrochloride intermediate alpha-6-doxycycline through hydrogenation on basis of silica gel rhodium catalyst
A technology of doxycycline hydrochloride and deoxyoxytetracycline, applied in chemical instruments and methods, preparation of organic compounds, physical/chemical process catalysts, etc., can solve the problems of high process cost, high cost, large amount of catalyst, etc. Achieve high catalytic activity and selectivity, facilitate industrial production, and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of silica gel rhodium catalyst
[0027] (1) Silica gel activation
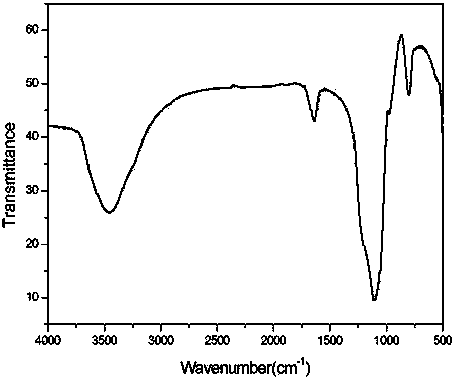
[0028] 3g of silica gel (10-100μm) was calcined and activated in a tube furnace at a heating rate of 2-10°C / min, raised to 120-150°C and kept for 1-5 hours to obtain 2.99g of activated silica gel. Infrared spectrum see figure 1 .
[0029] (2) De-ethanol
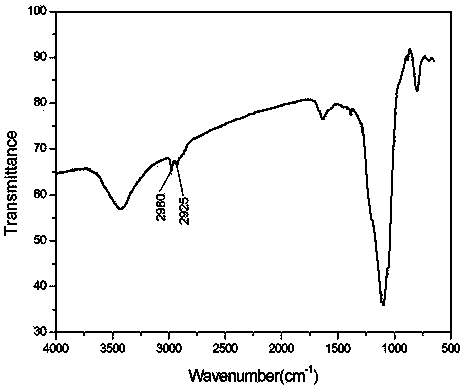
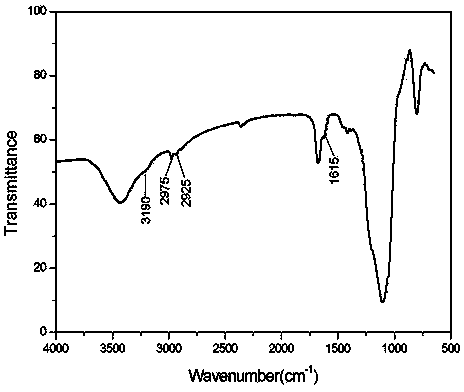
[0030] Add 2.99g of activated silica gel and 100ml of tetrahydrofuran into a three-necked flask, ventilate with nitrogen, stir at room temperature for 10-30min, add 6.0g of 3-aminopropyltriethoxysilane, stir at 40°C for 5-7 hours, filter with 10ml The filter cake was rinsed with tetrahydrofuran, and then vacuum-dried at 40° C. for 10 h to obtain 4.25 g of functionalized silica gel. The infrared spectrum of the resulting solid is shown in figure 2 . (3190cm -1 :NH 2 Symmetric stretching vibration, 2980cm -1 :CH 2 Antisymmetric stretching vibration, 2925cm -1 :CH 2 Symmetric stretching vibration, 1615cm -1 :NH 2 Scissor vibrati...
Embodiment 2
[0036] Preparation of silica gel rhodium catalyst
[0037] (1) Silica gel activation
[0038] 3g of silica gel (10-100μm) was calcined and activated in a tube furnace at a heating rate of 2-10°C / min, raised to 280-300°C and kept for 1-5 hours to obtain 2.99g of activated silica gel.
[0039] (2) De-ethanol
[0040] Add 2.99g of activated silica gel and 100ml of tetrahydrofuran into a three-necked flask, ventilate with nitrogen, stir at room temperature for 10-30min, add 1.5g of 3-aminopropyltriethoxysilane, stir for 4 hours at 20°C, filter with suction, and rinse with 10ml of tetrahydrofuran The filter cake was washed, and then vacuum-dried at 40° C. for 10 h to obtain 4.25 g of functionalized silica gel.
[0041] (3) Rhodium complex RhH(CO)[P(C 6 h 5 ) 3 ] 3 preparation of
[0042] Dissolve 0.257g rhodium trichloride trihydrate in 10ml ethanol, dissolve 2.53g triphenylphosphine in 100ml ethanol, mix and stir at room temperature for 15-20min, dissolve 0.75g potassium h...
Embodiment 3
[0046] Preparation of silica gel rhodium catalyst
[0047] (1) Silica gel activation
[0048] 3g of silica gel (10-100μm) was calcined and activated in a tube furnace at a heating rate of 2-10°C / min, raised to 200-250°C and kept for 4-5 hours to obtain 2.99g of activated silica gel.
[0049] (2) De-ethanol
[0050] Add 2.99g of activated silica gel and 100ml of tetrahydrofuran into a three-necked flask, blow nitrogen, stir at room temperature for 10-30min, add 9.0g of 3-aminopropyltriethoxysilane, stir at 70°C for 14 hours, filter with suction, and rinse with 10ml of tetrahydrofuran The filter cake was washed, and vacuum-dried at 40° C. for 10 h to obtain 4.25 g of functionalized silica gel.
[0051] (3) Rhodium complex RhCl(CO)[P(C 6 h 5 ) 3 ] 3 preparation of
[0052] Take 3.5g [Rh(CO) 2 Cl] 2 Dissolve in 10ml of chloroform, take 15g of triphenylphosphine and dissolve in 100ml of chloroform, stir at room temperature, drop the triphenylphosphine solution into the pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com