Alpha-cyano-group-4-hydroxycinnamic acid normal propyl ester, preparation method and application
A technology of hydroxycinnamic acid and n-propyl ester, which is applied in the preparation of carboxylic acid nitriles, chemical instruments and methods, and the preparation of organic compounds. It can solve the problems of low mass spectrometry analysis and detection, sample loss, and high prices of commercial products. The effect of reducing requirements and improving test results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
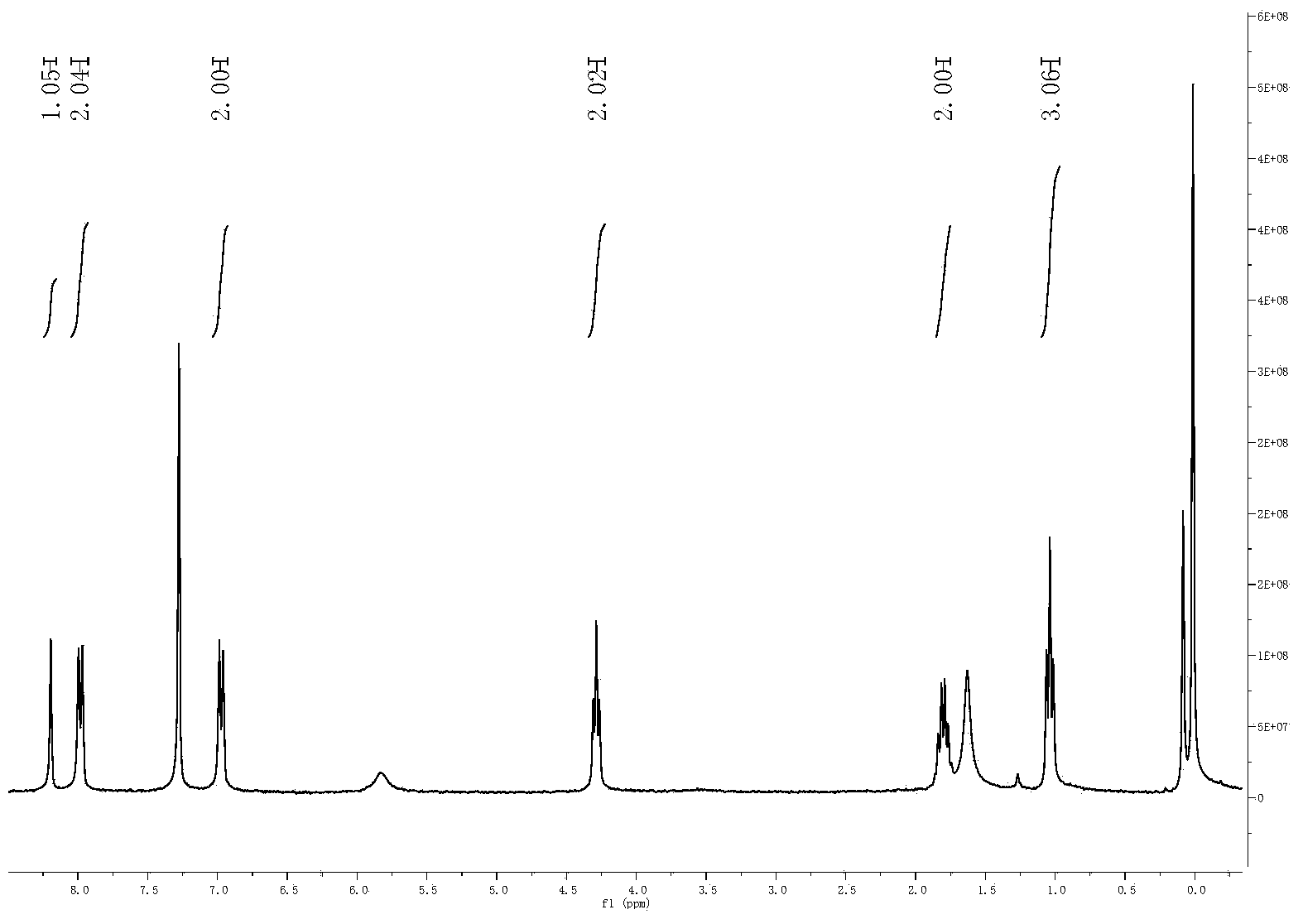
[0041] Example 1: Synthesis of n-propyl α-cyano-4-hydroxycinnamate
[0042] Add 0.2 mL piperidine to ethanol (30 mL), then add propyl cyanoacetate (0.6357 g, 5 mmol) and 4-hydroxybenzaldehyde (0.6717 g, 5.5 mmol). After the solid drug was dissolved, toluene (30 mL) was added, and the temperature was raised to 130° C., stirred and refluxed to remove water for 3 hours to stop the reaction. After cooling to room temperature, it was concentrated to 10 mL by rotary evaporation. The obtained concentrated liquid was poured into 300 mL of primary water, and solids were precipitated, and a slightly yellow powder filter cake was obtained by suction filtration. The filter cake was recrystallized once with ethanol (50 mL) to obtain the slightly yellow product α-cyano-4-hydroxycinnamic acid n-propyl ester (0.7958 g, 0.34 mmol), and the yield was 68.82%.
[0043] The structure identification data of α-cyano-4-hydroxycinnamic acid n-propyl ester are as follows:
[0044] 1 H NMR(300MHz, CDCl 3 )δ...
Embodiment 2
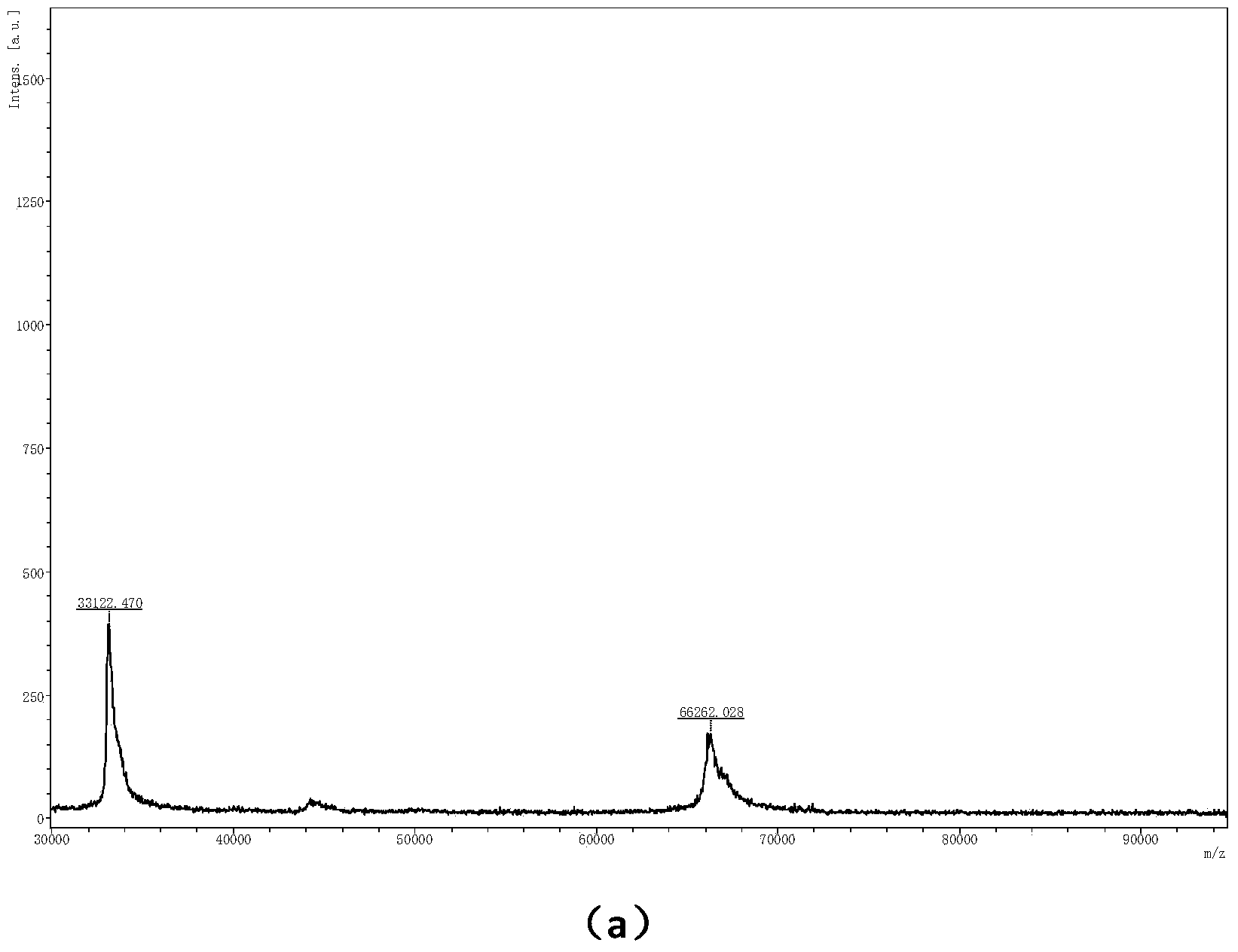
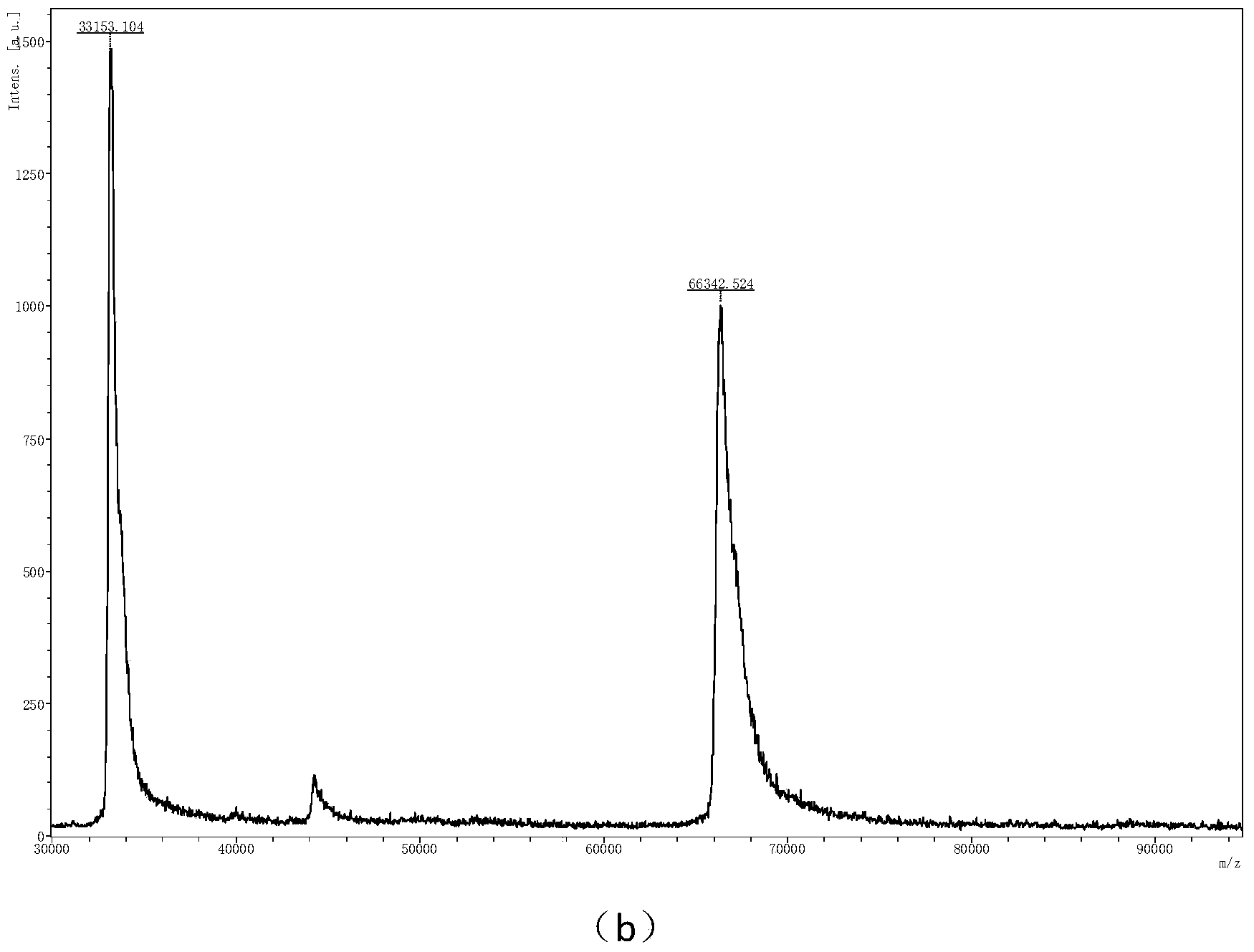
[0046] Example 2: Using α-cyano-4-hydroxycinnamic acid n-propyl ester as a matrix to perform mass spectrometry analysis on protein standards.
[0047] Take various prepared sample solutions (respectively bovine serum albumin (BSA), equine myoglobin (Myoglobin) and bovine insulin (Insulin) standards, all purchased from sigma company, the concentration is 1μM) and matrix solution ( Concentration is 5mM) Mix uniformly at a volume ratio of 1:1, then add 1μL of mixed sample to the MALDI target plate, place it in the air to volatilize the solvent, and then perform mass spectrometry analysis.
[0048] Compared with the traditional matrix α-cyano-4-hydroxycinnamic acid test sample, α-cyano-4-hydroxycinnamic acid n-propyl ester is used as the matrix for BSA ( figure 2 b, m / z66342, [M+H] + ;33153,[2M+H] + ), Myoglobin ( image 3 b, m / z16960.817,[M+H] + ;8479.374,[2M+H] + ,5650.476,[3M+H] + ), Insulin ( Figure 4 b, m / z5734.439) have obtained relatively high signal intensity, so it has better...
Embodiment 3
[0049] Example 3: Mass spectrometry analysis of polypeptide standards.
[0050] Take [Gly 14 ]-Humanin G human (amino acid sequence is Met-Ala-Pro-Arg-Gly-Phe-Ser-Cys-Leu-Leu-Leu-Leu-Thr-Gly-Glu-Ile-Asp-Leu-Pro-Val-Lys -Arg-Arg-Ala, with a concentration of 1μM, purchased from sigma company) The standard solution and matrix solution (with a concentration of 5mM) are mixed uniformly in a volume ratio of 1:1, and then 1μL of the mixed sample is added to the MALDI target plate and placed in the air The solvent is evaporated, and then mass spectrometry is performed.
[0051] Compared with the traditional matrix α-cyano-4-hydroxycinnamic acid test sample, α-cyano-4-hydroxycinnamic acid n-propyl ester is used as the matrix for [Gly 14 ]-Humanin G human( Figure 5 b, m / z2685.761,[M+H] + ;1343.516,[2M+H] + ) Have obtained relatively high signal intensity, and thus have better matrix performance. The amount of the analyte in the figure is 0.5 pmol, which shows that the sensitivity of the ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com