Method for detecting multiple residual solvents in medicament
A drug and detector technology, which is applied in the detection field of gas chromatography, can solve the problem that the detection method cannot meet the demand, and achieves the effects of low accuracy requirements, simple operation and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
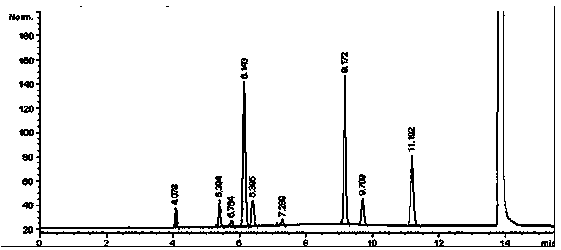
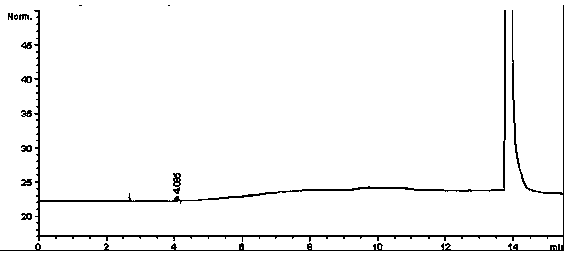
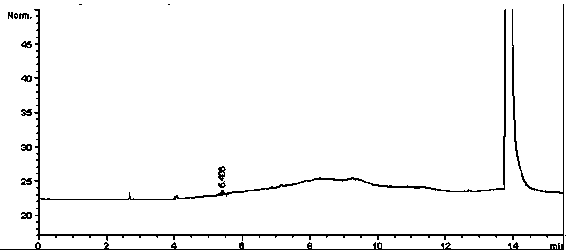
[0063] 1 Instruments and reagents
[0064] Agilent6890N gas chromatograph; Agilent7694E headspace sampler; cefotaxime sodium (batch number: TS0022); DMSO is HPLC grade, and other reagents are of analytical grade.
[0065] 2 Chromatographic conditions
[0066] Chromatographic column: capillary column with 100% polydimethylsiloxane as stationary liquid (HP-1 60m×530μm×5.00μm);
[0067] Carrier gas: N 2 ;
[0068] Ascending heating: keep at 40°C for 4 minutes, then start at 20°C˙min -1 The temperature is raised to 150°C at a rate of 6 minutes;
[0069] The inlet temperature is 200°C (split ratio: 1:1, column head pressure 7.70psi);
[0070] Detector: hydrogen flame ionization detector (FID);
[0071] Detector temperature: 250°C;
[0072] Headspace equilibrium temperature: 60°C;
[0073] Headspace equilibration time: 30min;
[0074] With DMSO as solvent.
[0075] 3 Preparation of solution
[0076] 3.1 Preparation of reference substance stock solution
[0077] Acc...
Embodiment 2
[0107] 1 Instruments and reagents
[0108] Agilent6890N gas chromatograph; Agilent7694E headspace sampler; meropenem sodium (batch number: FP005); DMSO was HPLC grade, and other reagents were of analytical grade.
[0109] 2 Chromatographic conditions
[0110] Chromatographic column: capillary column with 100% polydimethylsiloxane as stationary liquid (HP-1 60m×530μm×5.00μm);
[0111] Carrier gas: N 2 ;
[0112] Ascending heating: keep at 40°C for 8 minutes, raise the temperature to 150°C at a rate of 20°C / min, and hold for 8 minutes;
[0113] The inlet temperature is 200°C (split ratio: 1:1, column head pressure 7.70psi);
[0114] Detector: hydrogen flame ionization detector (FID);
[0115] Detector temperature: 250°C;
[0116] Headspace equilibrium temperature: 60°C;
[0117] Headspace equilibration time: 30min;
[0118] With DMSO as solvent.
[0119] Other steps are identical with embodiment 1.
[0120] 3 Solvent test results
[0121] Accurately measure ...
Embodiment 3
[0124] 1 Instruments and reagents
[0125] Agilent6890N gas chromatograph; Agilent7694E headspace sampler; cefoxitin (batch number: TX011); DMSO is HPLC grade, and other reagents are of analytical grade.
[0126] 2 Chromatographic conditions
[0127] Chromatographic column: capillary column with 100% polydimethylsiloxane as stationary liquid (HP-1 60m×530μm×5.00μm);
[0128] Carrier gas: N 2 ;
[0129] Ascending heating: keep at 40°C for 6 minutes, raise the temperature to 150°C at a rate of 20°C / min, and hold for 5 minutes;
[0130] The inlet temperature is 200°C (split ratio: 1:1, column head pressure 7.70psi);
[0131] Detector: hydrogen flame ionization detector (FID);
[0132] Detector temperature: 250°C;
[0133] Headspace equilibrium temperature: 60°C;
[0134] Headspace equilibration time: 30min;
[0135] With DMSO as solvent.
[0136] Other steps are identical with embodiment 1.
[0137] 3 Solvent test results
[0138] Precisely measure the cefoxiti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com