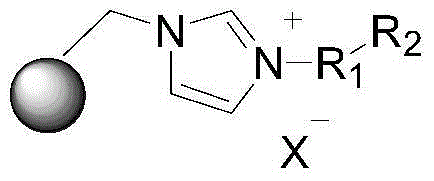
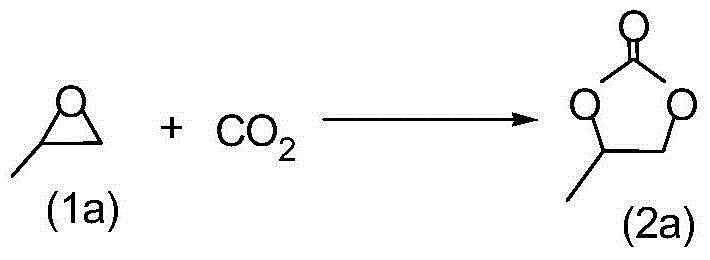Supported ionic liquid catalyst, as well as preparation and application thereof
An ionic liquid and catalyst technology, applied to the supported ionic liquid catalyst and its preparation and application fields, can solve the problems of difficult purification and separation of products and catalysts, harsh reaction conditions, inconvenient recycling, etc., and achieve thermal stability and chemical stability. Good, simple preparation, pore structure controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Chloromethylation of organic mesoporous materials
[0024] Mix 5g of FDU-type mesoporous phenolic resin with 60ml of chloromethyl ether at -5°C under the protection of argon, then weigh 21g of anhydrous aluminum trichloride and add it to the above mixture three times. The time is 0.5 hours. Stir at room temperature for 12 hours to carry out chloromethylation reaction. After the reaction, add an appropriate amount of pure water to completely hydrolyze aluminum trichloride, then cool to 0°C and filter. The filtrate is mixed with 500ml pure water and 200ml acetone Alternately washing to obtain a brown solid, and after vacuum drying, a chloromethylated FDU organic mesoporous material was obtained.
[0025] (2) Imidazolization of organic mesoporous materials
[0026] After mixing 5g of chloromethylated FDU mesoporous phenolic resin with 60ml of acetonitrile solution, add 4g of imidazole to carry out imidazole reaction at 80°C for 12 hours. After the reaction, the filtra...
Embodiment 2
[0030] (1) Chloromethylation of organic mesoporous materials
[0031] Mix 5g of FDU-type mesoporous phenolic resin with 60ml of chloromethyl ether at -5°C under the protection of argon, then weigh 21g of anhydrous aluminum trichloride and add it to the above mixture three times. The time is 0.5 hours. Stir at room temperature for 12 hours to carry out chloromethylation reaction. After the reaction, add an appropriate amount of pure water to completely hydrolyze aluminum trichloride, then cool to 0°C and filter. The filtrate is mixed with 500ml pure water and 200ml acetone Alternately washing to obtain a brown solid, and after vacuum drying, a chloromethylated FDU organic mesoporous material was obtained.
[0032] (2) Imidazolization of organic mesoporous materials
[0033] After mixing 5g of chloromethylated FDU mesoporous phenolic resin with 60ml of acetonitrile solution, add 4g of imidazole to carry out imidazole reaction at 80°C for 12 hours. After the reaction, the filtra...
Embodiment 3
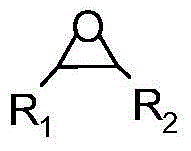
[0037] 1.75g (30mmol) propylene oxide (1a) and 0.28g mesoporous phenolic resin supported 3-hydroxyethylimidazolium bromide salt ionic liquid catalyst (R 1 =(CH 2 ) 2 , R 2 =OH) Fill the autoclave with CO 2 , and then heated to 120°C to carry out the cycloaddition reaction of the following structural formula,
[0038]
[0039] The reaction pressure is 1MPa, during the reaction CO 2 The pressure was kept constant, and the reaction time was 4 hours. After the reaction, the reactor was cooled to 0°C, and excess carbon dioxide gas was slowly released, and then the reaction liquid was filtered. The filtrate was analyzed qualitatively by GC-MS, and 0.2 g of biphenyl was added as The standard substance was quantitatively analyzed by gas chromatography, and the product obtained was propylene carbonate (2a), with a yield of 99.0% and a selectivity of 99.8%. The filtered catalyst can be recycled after being washed with acetone and dried.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com