Rigid aromatic diamine monomer as well as preparation method and application thereof
A technology for aromatic diamines and monomers, which is applied in the field of rigid aromatic diamine monomers. It can solve the problems of being in the initial stage and few types of monomers, and achieve excellent film-forming properties, easy separation and purification, and simple synthesis routes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
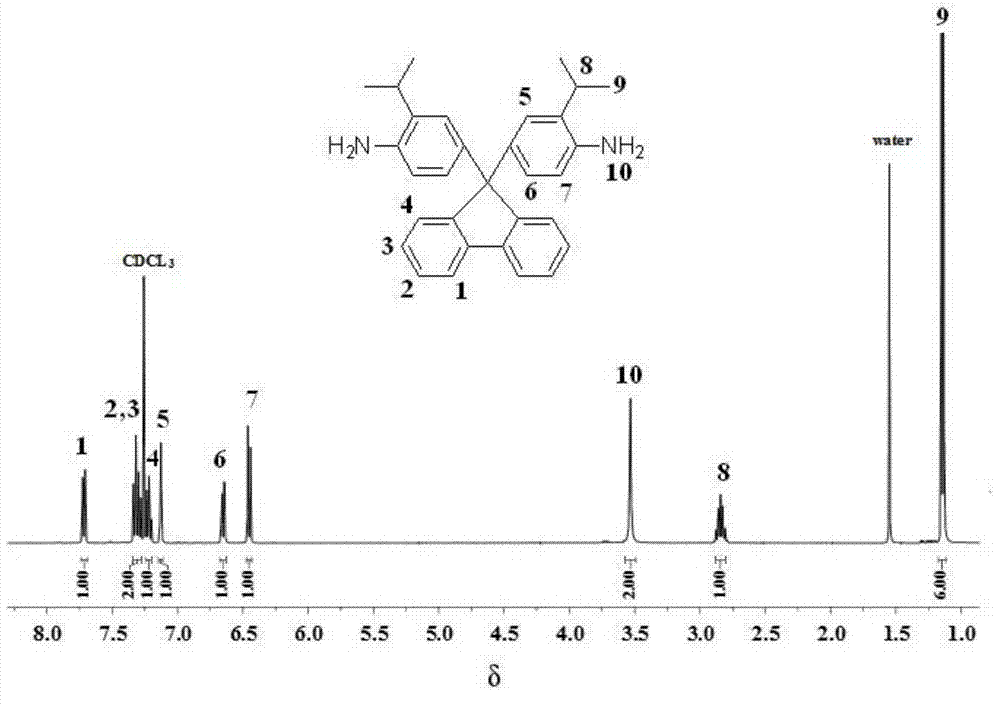
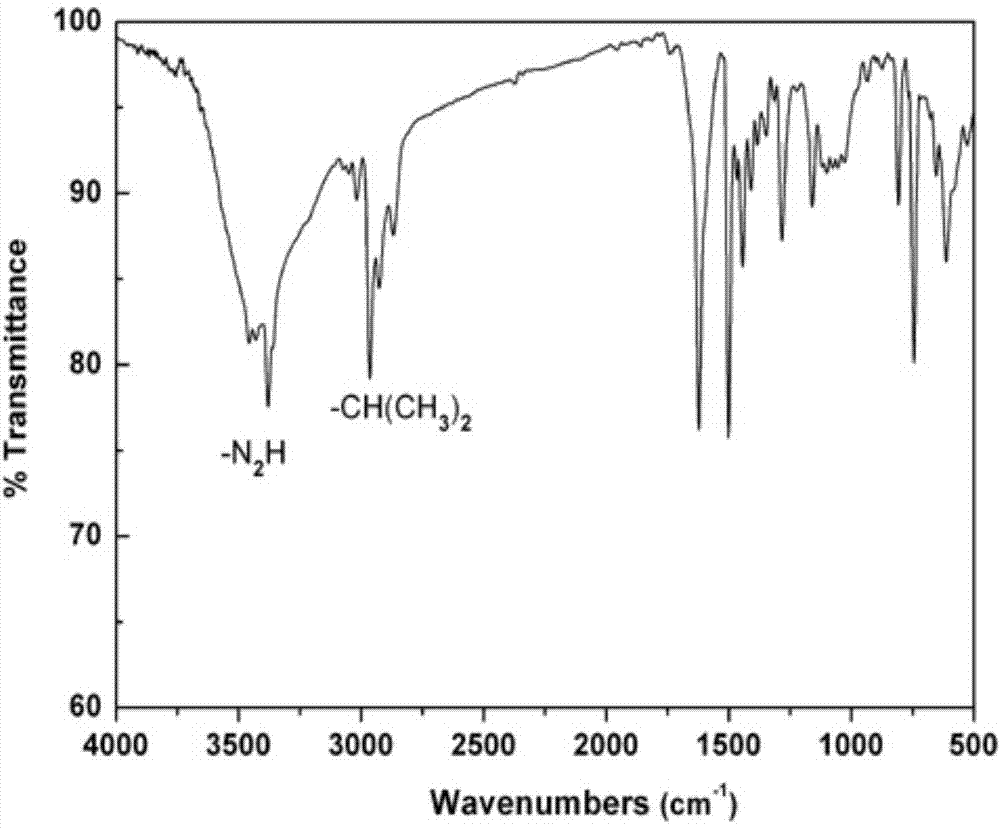
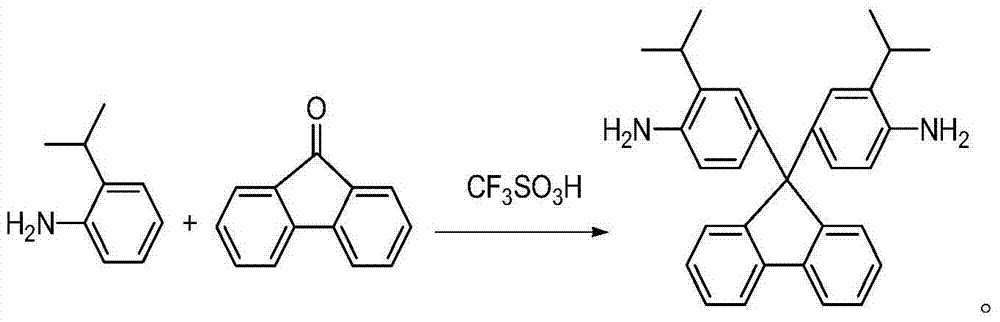
[0021] A preparation method of rigid aromatic diamine monomer 9,9-bis(4-amino-3-isopropylphenyl) fluorene has the following preparation steps:
[0022] (1) Under the protection of nitrogen, add 18.02g (0.10mol) of 9-fluorenone and 40.56g (0.3mol) of 2-isopropylaniline into a 250ml three-necked flask, stir at room temperature until 9-fluorenone is dissolved , And then slowly add 4.5g (0.03mol) of trifluoromethanesulfonic acid, continue to stir and react for 5h under reflux state, and end the reaction;
[0023] (2) After the reaction product of step (1) is brought to room temperature, it is neutralized to neutrality with 10% sodium hydroxide solution, and the reaction product is transferred to a mixed solvent of ethanol and water (the volume ratio of ethanol and water is 5: 1) Fully agitate, precipitate, filter with suction, and further recrystallize with toluene to obtain a white powdery solid, which is a rigid aromatic diamine monomer containing a fluorenyl structure and a diisopro...
Embodiment 2
[0028] A preparation method of rigid aromatic diamine monomer 9,9-bis(4-amino-3-isopropylphenyl) fluorene has the following preparation steps:
[0029] (1) Under the protection of nitrogen, add 18.02g (0.10mol) of 9-fluorenone and 28.97g (0.214mol) of 2-isopropylaniline into a 250ml three-necked flask, stir at room temperature and wait until 9-fluorenone is dissolved , And then slowly add 3.0g (0.02mol) of trifluoromethanesulfonic acid, continue to stir and react under reflux for 10h, and end the reaction;
[0030] (2) After the reaction product of step (1) is brought to room temperature, it is neutralized with a 10% sodium carbonate solution, and the reaction product is transferred to a mixed solvent of ethanol and water (the volume ratio of ethanol and water is 3:1 ), fully agitate, precipitate, filter with suction, and further recrystallize with ethanol to obtain a white powdery solid, that is, a rigid aromatic diamine monomer containing a fluorenyl structure and a diisopropyl g...
Embodiment 3
[0033] A preparation method of 9,9-bis(4-amino-3-isopropylphenyl) fluorene-based polyaramid has the following steps:
[0034] Under the protection of nitrogen, 3mmol 9,9-bis(4-amino-3-isopropylphenyl) fluorene and 3mmol aromatic diacid (terephthalic acid, isophthalic acid, diphenyl ether acid or hexafluoroiso One of propyl diacid) was added to a 50ml three-necked flask, and then 6-9mmol triphenyl phosphite, 6-9mmol pyridine, 0.3-0.6g anhydrous calcium chloride and 12-16ml N- Methylpyrrolidone, stir at room temperature until the reactants are completely dissolved, then heat to 100~120℃, stir and react for 4~8h, when the system becomes viscous, stop the reaction, pour the reaction solution into ethanol, and obtain a white fibrous shape The polymer was further washed twice with hot water and dried under vacuum at 120°C to obtain polyaramid polymer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com