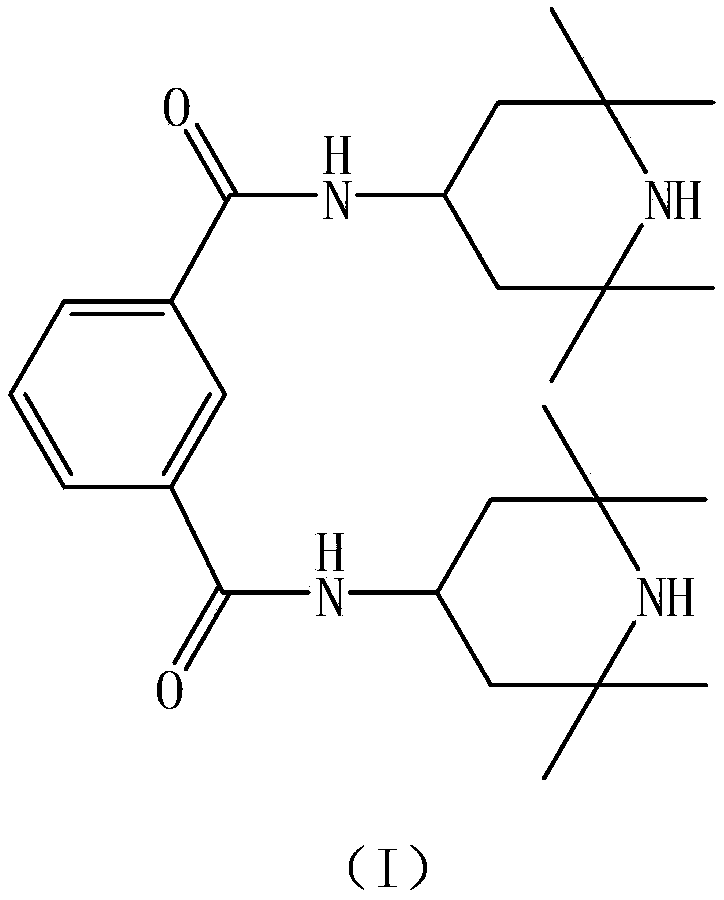
Preparation method of N, N'-bis(2, 2, 6, 6-tetramethyl-4-piperidyl)-1, 3-benzenedicarboxamide
A technology of phthalamide and isophthaloyl chloride, which is applied in the field of hindered amine light stabilizers, can solve the problems of many organic substances, ineffective utilization of raw materials, and other problems, achieves mild reaction conditions, is conducive to full utilization, The effect of reducing unit consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
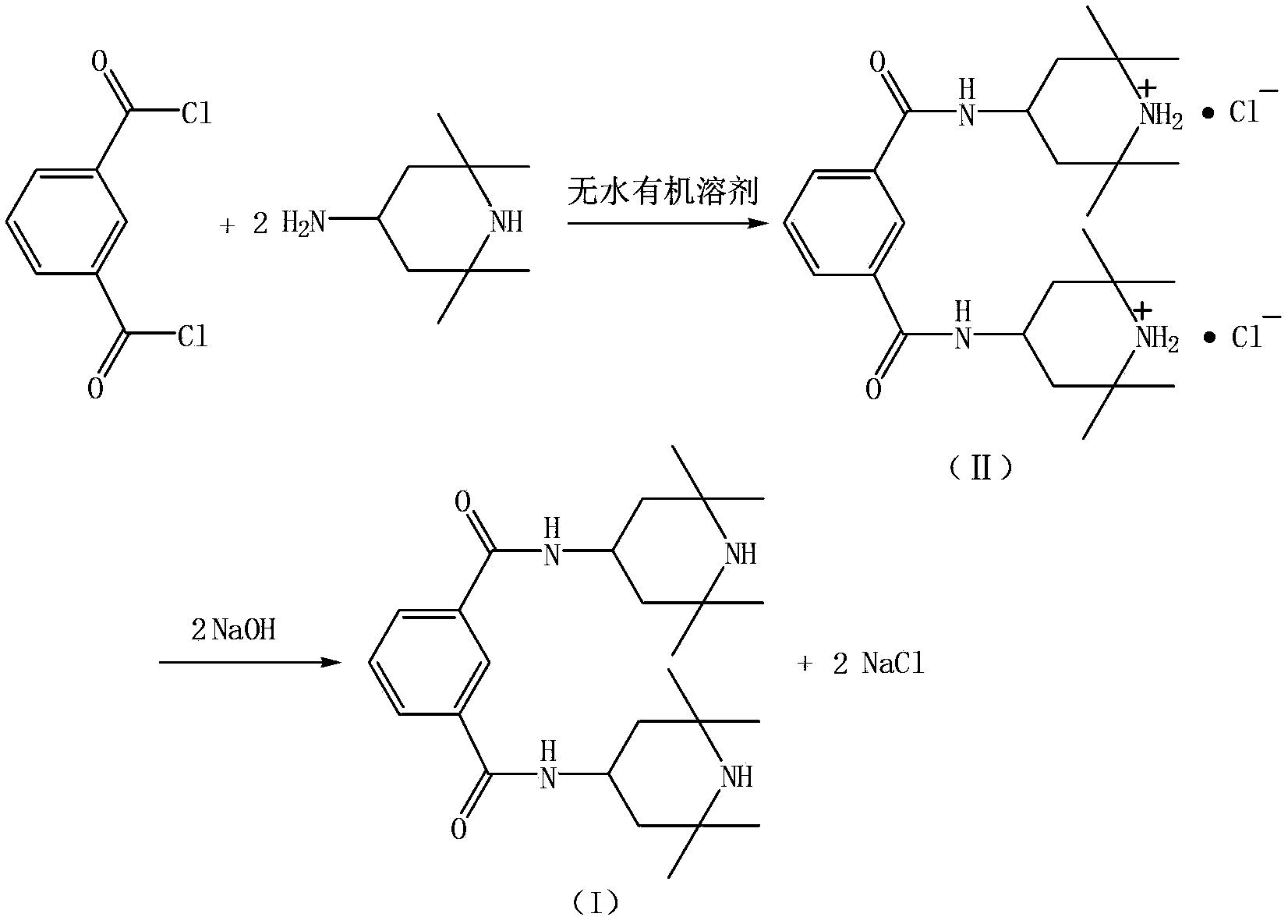
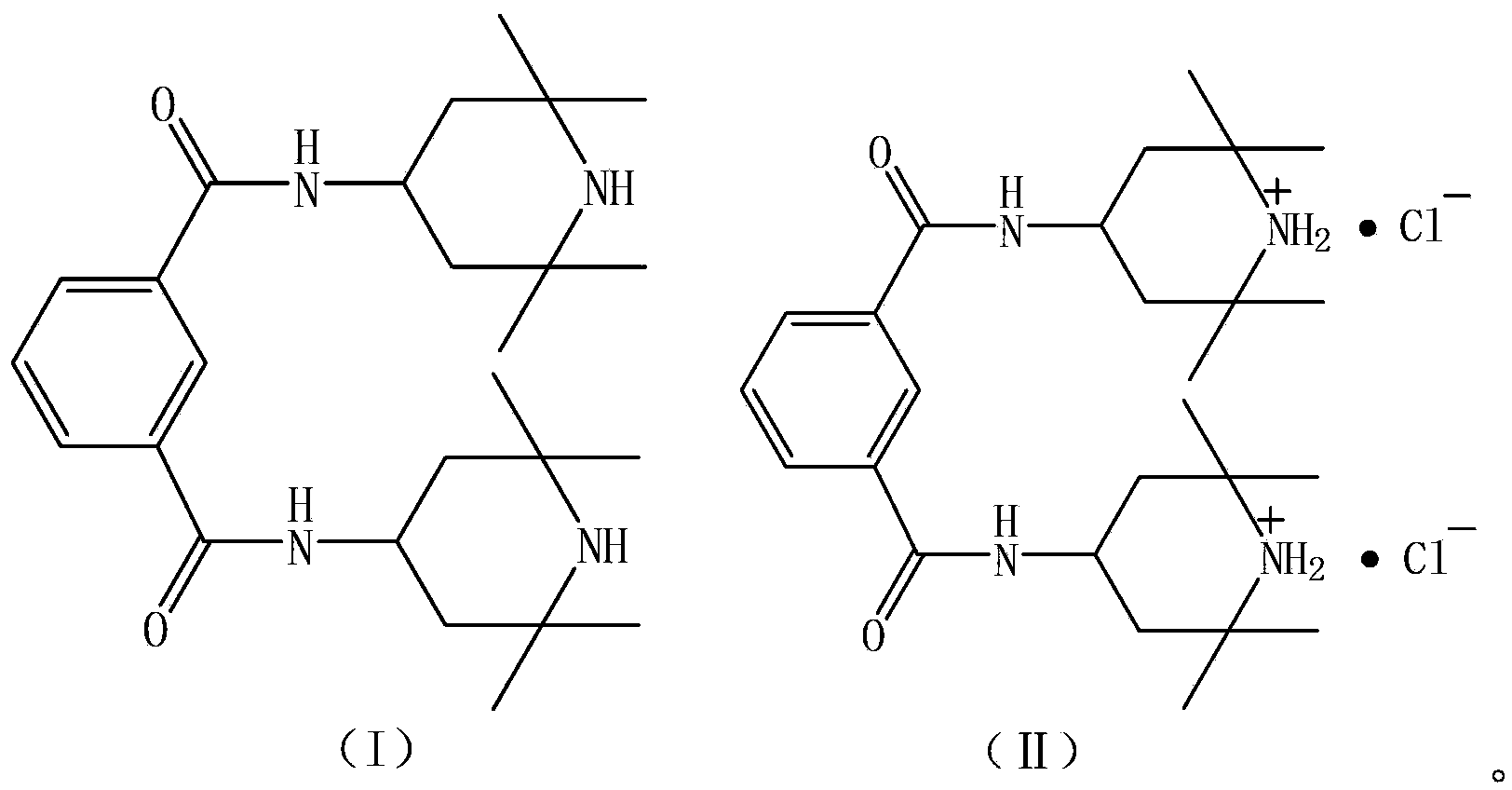
[0025] Put 700kg of methyl ethyl ketone and 184.4kg of 2,2,6,6-tetramethyl-4-aminopiperidine (according to the purity of 99%, 1.17kmol) into the reactor, stir and cool down to 0°C, slowly drop into the molten 92.3kg of phthaloyl chloride (99% purity, 0.45kmol) was kept at a reaction temperature of 10°C, and the reaction was incubated for 5 hours after the dropwise addition was completed. Centrifuge with a fully automatic closed centrifuge, wash the filter cake with anhydrous methyl ethyl ketone, collect the mother liquor and washing liquid for the next batch of production, and vacuum-dry the filter cake to obtain 227kg of intermediate (Ⅱ), with a yield of 98.7% (calculated as isophthaloyl chloride).
[0026] Put 227kg of the obtained intermediate (II) and 620kg of water into the reaction kettle and stir evenly. The reaction temperature is 25-30°C. Add about 125kg of 30% sodium hydroxide aqueous solution (0.94kmol) dropwise until the pH value of the reaction solution is 10, and...
Embodiment 2
[0028] Put 950kg of recovered mother liquor and 140.4kg of 2,2,6,6-tetramethyl-4-aminopiperidine (99% purity, 0.9kmol) into the reaction kettle, stir and cool down to 0°C, slowly drop Add 92.3kg of molten isophthaloyl chloride (0.45kmol based on 99% purity) to keep the reaction temperature at 15°C. After the dropwise addition, keep the reaction for 1 hour. Centrifuge with a fully automatic closed centrifuge, wash the filter cake with anhydrous methyl ethyl ketone, collect the mother liquor and washing liquid for the next batch of production, and vacuum dry the filter cake to obtain 228kg of intermediate (Ⅱ), with a yield of 99.6% (calculated as isophthaloyl chloride).
[0029] Put 228kg of the obtained intermediate (II) and 1200kg of water into the reaction kettle and stir evenly. The reaction temperature is 25-30°C. Add about 125kg of 30% sodium hydroxide aqueous solution (0.94kmol) dropwise until the pH value of the reaction solution is 10, and keep the reaction for 3 hours....
Embodiment 3
[0031]Put 1800kg of petroleum ether (60~90°C), 147.4kg of 2,2,6,6-tetramethyl-4-aminopiperidine (according to 99% purity, 0.945kmol) into the reactor, stir and cool down to -5°C , slowly dropwise added 92.3kg of molten isophthaloyl chloride (0.45kmol based on a purity of 99%) to keep the reaction temperature at 0°C, and kept the reaction for 5 hours after the dropwise addition was completed. Centrifuge with a fully automatic closed centrifuge, wash the filter cake with petroleum ether (60-90°C), collect the mother liquor and washing liquid for the next batch of production, and vacuum-dry the filter cake to obtain 228.8kg of intermediate (Ⅱ) , Yield 99.5% (based on isophthaloyl chloride).
[0032] Put 228.8kg of the obtained intermediate (II) and 450kg of water into the reaction kettle and stir evenly. The reaction temperature is 25-30°C. Add about 125kg of 30% sodium hydroxide aqueous solution (0.94kmol) dropwise until the pH value of the reaction solution is 10. Hours, centr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com