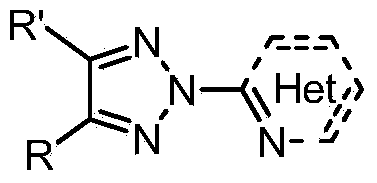
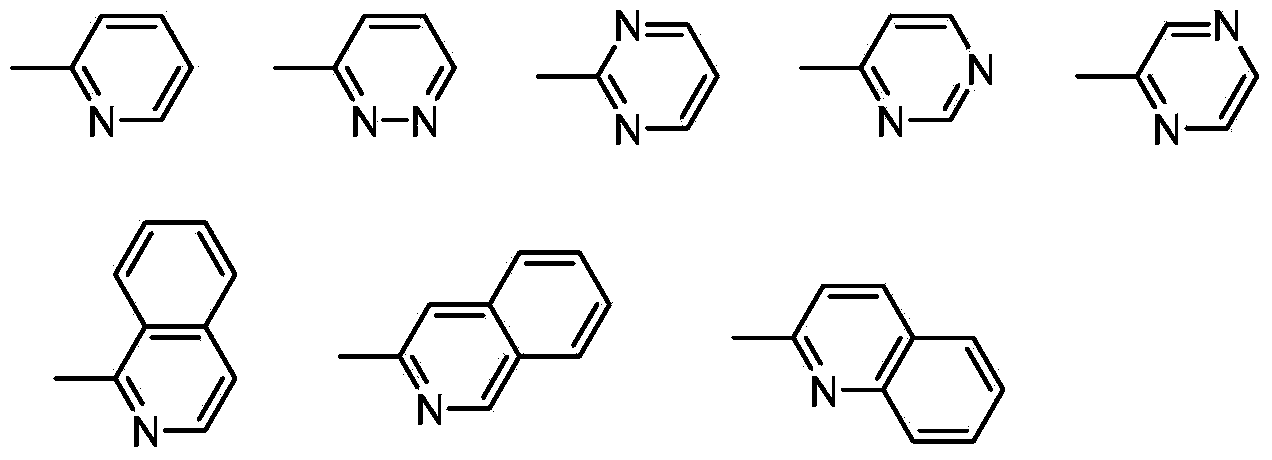
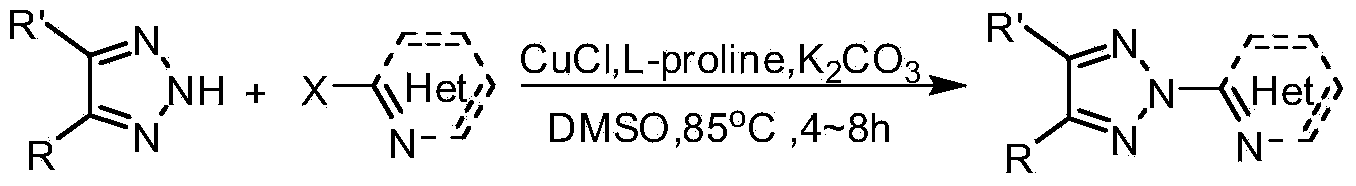N2-substituted-1,2,3-triazole ligand auxiliary Cu (I) catalysts and applications thereof
A catalyst and composite catalyst technology, applied in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, organic chemistry, etc., can solve the problems of long reaction time, difficult synthesis, easy salt disproportionation, etc. Simple synthesis, strong substrate adaptability and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of N2 Substituted 1,2,3-Triazole Ligand 1
[0033]
[0034] Add NH-1,2,3-triazole (14.5g, 100mmoL), o-bromopyridine (17.4g, 110mmmoL), cuprous chloride (0.99g, 10mmoL), L-proline ( 2.3g, 20mmoL), potassium carbonate (20.7g, 150mmoL), DMSO (180mL), protected by argon, and heated in an oil bath at 85-95°C for about 6 hours to complete the reaction. After the reaction was completed, it was extracted with ethyl acetate, and the organic phase was washed with anhydrous Na 2 SO 4 Drying, filtration and concentration to obtain the crude product was separated and purified by thin-layer silica gel column chromatography to obtain a white solid, 13.5g, with a yield of 61%. The structure of this compound is characterized as follows: 1 H NMR (400MHz, CDCl 3 )δ8.57-8.67(m,1H),8.09-8.19(m,2H),7.84-7.99(m,3H),7.37-7.50(m,3H),7.35-7.29(m,1H).
[0035] Catalytic reaction
[0036]
[0037] Phenylacetylene (1.02g, 10mmoL), benzyl azide (1.47g, 11mmoL), N2 substituted ...
Embodiment 2
[0039] Synthesis of N2 Substituted 1,2,3-Triazole Ligand 2
[0040]
[0041] Add NH-1,2,3-triazole (1.45g, 10mmoL), o-bromopyrimidine (1.75g, 11mmoL), cuprous chloride (99mg, 1mmoL), L-proline (0.23 g, 2mmoL), potassium carbonate (2.07g, 15mmoL), DMSO (35mL), protected by argon, heated in an oil bath at 85-95°C for about 8 hours, the reaction was complete, and the reaction process was monitored by TLC. After the reaction, with ethyl acetate and NH 4 Cl solution extraction, organic phase with anhydrous Na 2 SO 4 Drying, filtration and concentration to obtain a crude product was separated and purified by thin-layer silica gel column chromatography to obtain a white solid, 1.23 g, with a yield of 55%. The structure of this compound is characterized as follows: 1 H NMR (400MHz, CDCl 3 )δ8.81(s,1H),8.52(d,J=4.0Hz,1H),8.25(d,J=8.0Hz,1H),7.85-8.00(m,2H),7.44-7.50(m,2H ),7.31-7.41(m,2H).
[0042] Catalytic reaction
[0043]
[0044] Add phenylacetylene (2.04g, 20mmoL), ...
Embodiment 3
[0049] Using the N2 substituted 1,2,3-triazole ligand 1 prepared in Example 1, investigate the catalytic reaction effect of Cu(I) salt and N2 substituted 1,2,3-triazole ligand 1 in different dosage ratios , taking the following reaction as an example, the results are shown in the table below.
[0050]
[0051] Add phenylacetylene (1.02g, 10mmoL), benzyl azide (1.47g, 11mmoL), 1,2,3-triazole ligand 1, CuI (see the table below for the amount) to a 50mL round-bottomed flask in sequence. Anhydrous methanol (25mL) was used as the reaction solvent, stirred at room temperature, and the treatment method was to filter and concentrate the reaction solution, and recrystallize to obtain the white solid 1,2,3-triazole product (see the table below for the yield).
[0052] The N2 substituted 1,2,3-triazole ligand 1 prepared in Example 1 assisted CuI to catalyze the reaction of phenylacetylene and benzyl azide under different catalytic amounts and temperatures as shown in the following tab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com