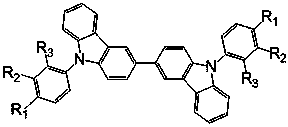
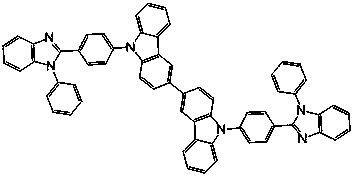
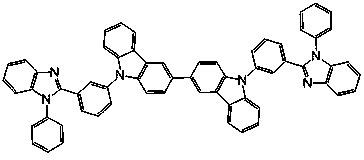
Bipolar phosphorescent host compounds, preparation method, application and electroluminescent device
An electroluminescent device and phosphorescent host technology, applied in the field of electroluminescent devices and bipolar phosphorescent host compounds, can solve the problem that the phosphorescent OLED cannot be well applied in industrial production, the efficiency of the phosphorescent light-emitting device cannot be further improved, and the adjacent Problems such as poor energy level matching of the active layer can achieve the effect of increasing the lifespan, reducing the degree of structural conjugation, and reducing the efficiency roll-off.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Preparation of bis(1-(N-phenylbenzimidazolyl)-3-(carbazolyl))benzene (DmBICP):
[0054] Dissolve 3-bromocarbazole (1.0 mmol), 3-pinacol borate carbazole (1.0 mmol) in toluene (50 ml) solution, and add 2M potassium carbonate aqueous solution (5.0 ml) and ethanol (5.0 ml ), and at the same time add the catalyst Pd(PPh3)4 (0.08 mmol), heated to 70 ~ 180 ℃ in the nitrogen environment, protected from light and refluxed for 3 ~ 48 hours; then cooled to room temperature, washed with water, extracted with dichloromethane, combined organic phase, dried over anhydrous sodium sulfate, filtered, removed the organic solvent, and recrystallized with dichloromethane and n-hexane to obtain a white solid powder, namely dicarbazole intermediate (0.8 mmol). Yield: 80.0%. MS (APCI): calcd for C 24 h 16 N 2 : 332.1, found, 333.4 (M+1) +
[0055] Dissolve the white solid powder (1.0 mmol) obtained in the previous step and the electron-withdrawing group halide 3-bromophenyl-N-phenylbenz...
Embodiment 2
[0057] Preparation of bis(1-(N-phenylbenzimidazolyl)-2-(carbazolyl))benzene (DoBICP):
[0058] A method similar to compound DmBICP was employed, except that 2-bromophenyl-N-phenylbenzimidazole was used instead of 3-bromophenyl-N-phenylbenzimidazole as the starting material in the Ullmann reaction. Bis(1-(N-phenylbenzimidazolyl)-2-(carbazolyl))benzene (DoBICP) white solid powder can be obtained, yield: 55%. MS (APCI): calcd for C 62 h 40 N 6 : 868.3, found, 869.5 (M+1) + .
Embodiment 3
[0060]Preparation of bis(1-(N-phenylbenzimidazolyl)-4-(carbazolyl))benzene (DpBICP):
[0061] A method similar to compound DmBICP was employed, except that 4-bromophenyl-N-phenylbenzimidazole was used as the starting material instead of 3-bromophenyl-N-phenylbenzimidazole in the Ullmann reaction. Bis(1-(N-phenylbenzimidazolyl)-4-(carbazolyl))benzene (DpBICP) white solid powder can be obtained, yield: 68%. MS (APCI): calcd for C 62 h 40 N 6 : 868.3, found, 869.2 (M+1) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com