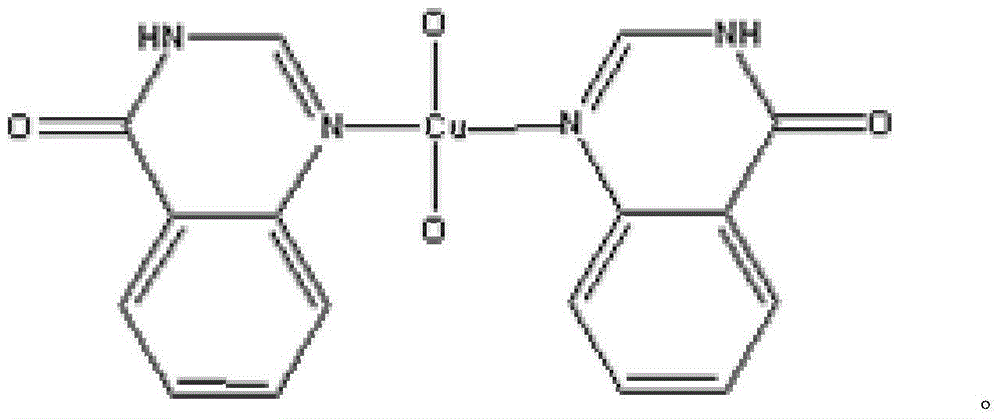
A kind of 4-(3h) quinazolone peroxy copper complex and its synthesis method
A quinazolidone copper peroxide and synthesis method technology, applied in the direction of copper organic compounds, etc., can solve the problems of harmfulness to human body and environment, low catalytic activity, long reaction time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation of 4-(3H) quinazolonone peroxycopper complex of the present invention:
[0022] Dissolve (0.0296g, 0.2mmol) 4-(3H)quinazolone and (0.0241g, 0.1mmol) copper nitrate tetrahydrate in 10ml of acetonitrile solvent, and pour the resulting mixed solution into polytetrafluoroethylene for high-pressure hydrothermal reaction In the kettle, under the hydrothermal reaction conditions of 150 and 130 degrees, react for one day respectively, and cool to room temperature. Filter and collect in a beaker to obtain a yellow filtrate. Put the filtrate in the air, 10-20 minutes, yellow crystals precipitated from the mother liquor. 0.0512 g of yellow 4-(3H)quinazolone peroxycopper complex crystals were collected, and the yield was about 66%.
Embodiment 2
[0024] The single crystal X-ray diffraction experiment conditions and results of the complex of the present invention are as follows:
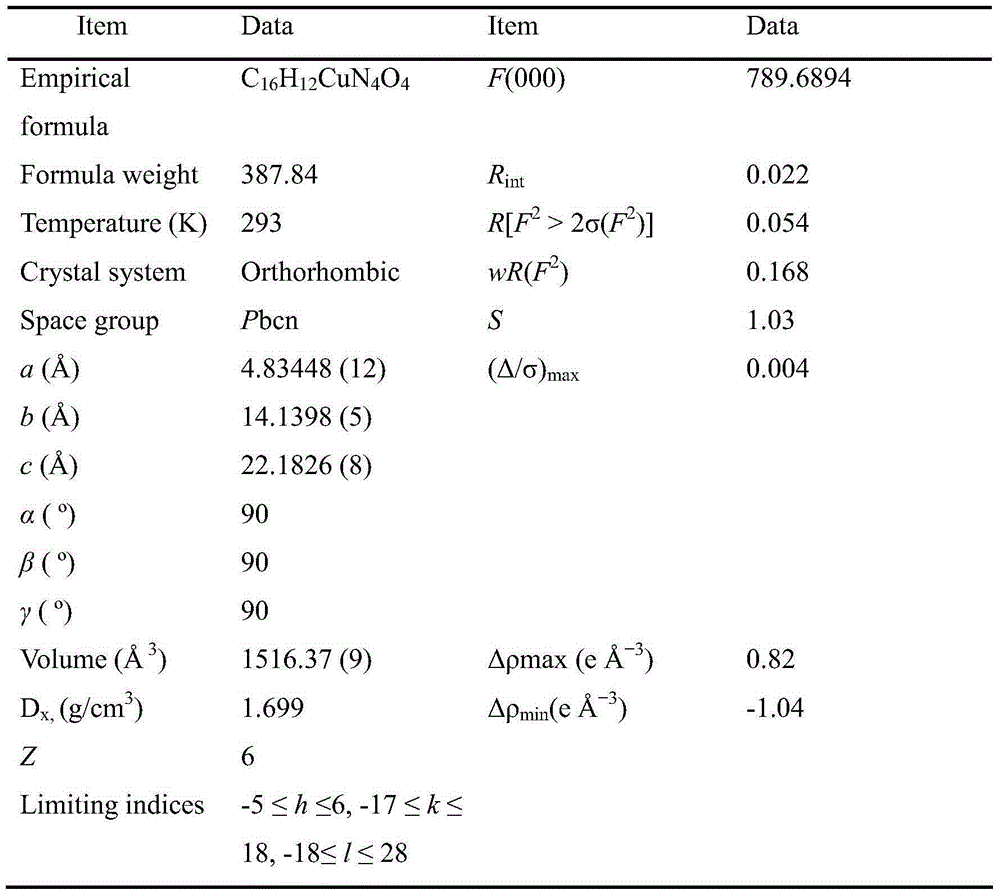
[0025] The single crystal X-ray diffraction experiment was carried out on a Bruker diffractometer, using a Mo target of a graphite monochromator, at a temperature of 293K, a voltage of 50kV, and a current of 80mA to collect independent diffraction points, using SHELXS-97 and SHELXL-97 program packages Carry out structure analysis, hydrogenation and refinement. All non-hydrogen atoms are refined using the full matrix least squares method (Full-matrix least-squares refinement base on F2). All non-hydrogen atoms are subjected to anisotropic refinement. Other details about the crystal The information is listed in Table 1, Table 2 and Table 3.
[0026] Table 1 Complex C 16 h 12 N 4 o 4 Cu unit cell and measurement parameters
[0027]
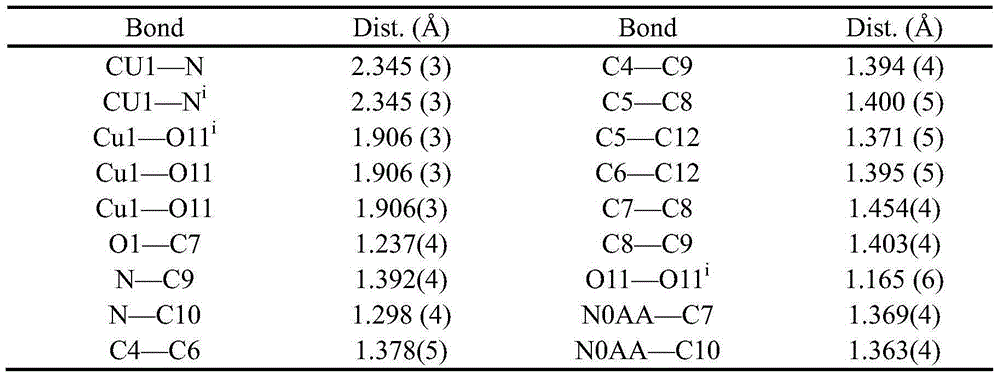
[0028] Table 2 Complex C 16 h 12 N 4 o 4 Cu bond length
[0029]
[0030] Table 3 Complexes [Cu(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com