Method for detecting pneumonic mycoplasma, quantum dot-labeled immunochromatographic test paper and preparation method thereof
An immunochromatographic test strip and Mycoplasma pneumoniae technology, applied in the field of medical immunodetection, can solve the problems of low sensitivity and low accuracy, and achieve the effects of good luminescence stability, narrow emission peak and symmetrical peak shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
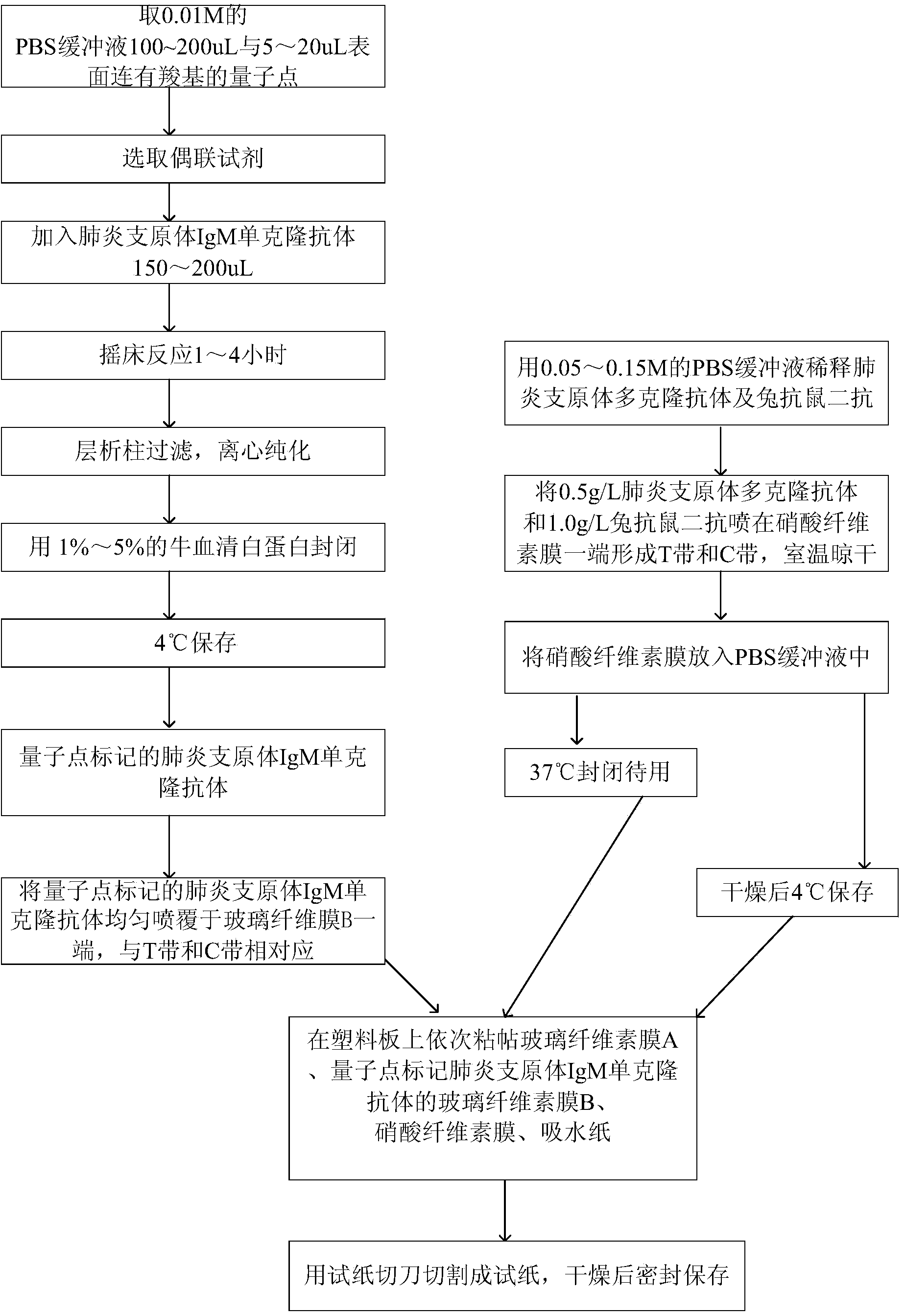
[0032] Embodiment 1: A kind of quantum dot-labeled immunochromatographic test paper is provided with plastic plate, nitrocellulose membrane, glass cellulose membrane A, quantum dot-labeled glass cellulose membrane B of mycoplasma pneumoniae IgM monoclonal antibody, absorbent paper, so The above-mentioned glass cellulose film A is the glass cellulose film purchased on the market without sample;
[0033] Wherein, the plastic plate is pasted with glass cellulose membrane A, quantum dot-labeled glass cellulose membrane B of mycoplasma pneumoniae IgM monoclonal antibody, nitrocellulose membrane, and absorbent paper in sequence;
[0034] Wherein, one end of the nitrocellulose membrane has a Mycoplasma pneumoniae polyclonal antibody and a rabbit anti-mouse secondary antibody to form a detection zone T and a quality control zone C;
[0035] Wherein, the quantum dot-labeled Mycoplasma pneumoniae IgM monoclonal antibody is located at the other end of the glass cellulose membrane B, corr...
Embodiment 2
[0041] Embodiment 2: the preparation method of test paper as mentioned above, as figure 1 shown, including the following steps:
[0042] (1) Coupling of quantum dots and Mycoplasma pneumoniae IgM monoclonal antibody:
[0043] Take 100-200uL of 0.01M PBS buffer and 5-20uL of quantum dots with carboxyl groups on the surface;
[0044] A coupling reagent is selected, and the coupling reagent is selected from hydroxysulfosuccinimide, 1-(3-dimethylaminopropyl)-3 ethylcarbodiamine hydrochloride;
[0045] Add 150-200 uL of Mycoplasma pneumoniae IgM monoclonal antibody;
[0046] Shaker reaction for 1 to 4 hours;
[0047] Column filtration, centrifugal purification;
[0048] Block with 1% to 5% bovine serum albumin;
[0049] Store at 4°C;
[0050] (2) Preparation of test paper:
[0051] Dilute the Mycoplasma pneumoniae polyclonal antibody and rabbit anti-mouse secondary antibody with 0.05-0.15M PBS buffer, spray 0.5g / L Mycoplasma pneumoniae polyclonal antibody and 1.0g / L rabbit a...
Embodiment 3
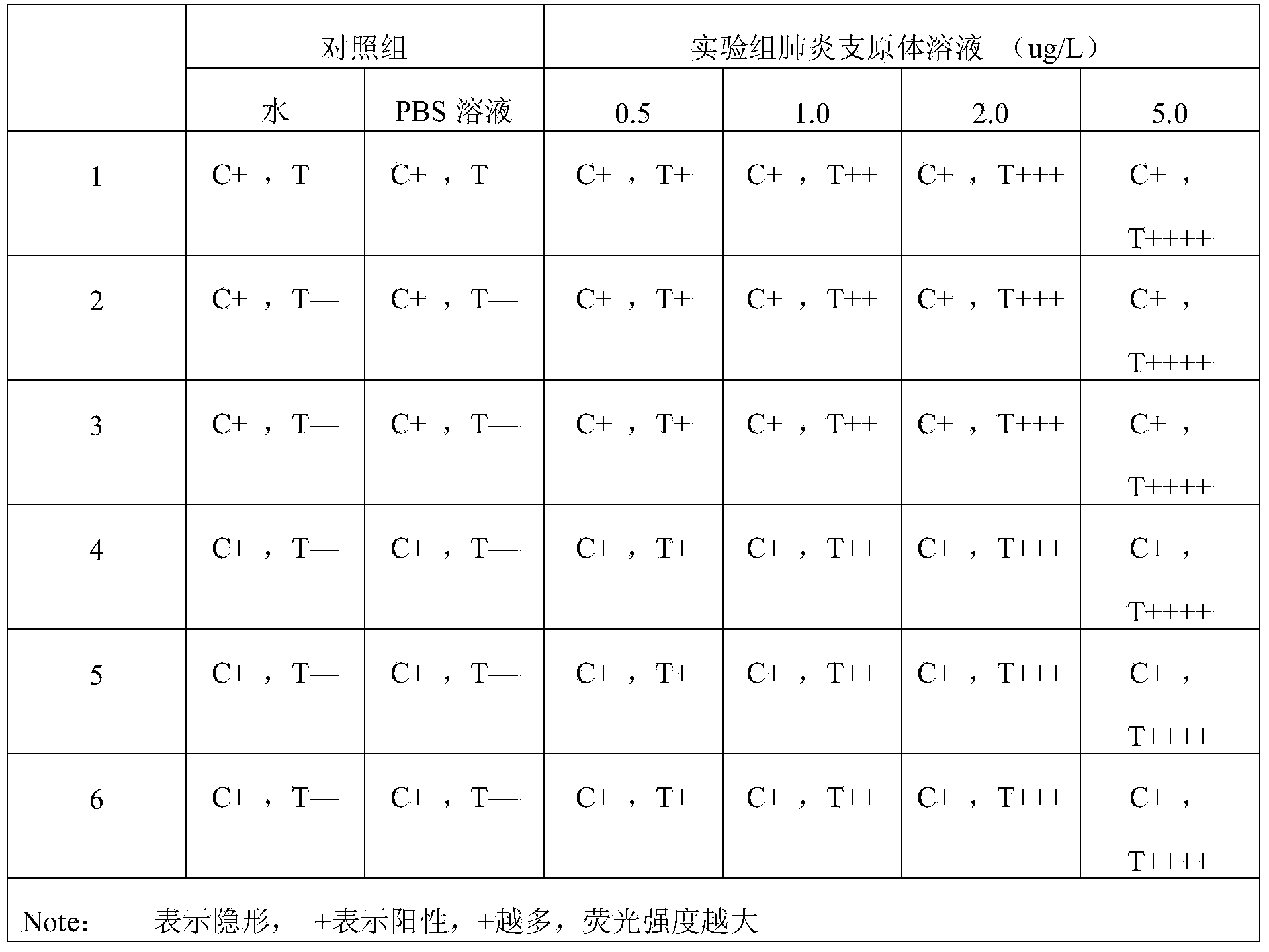
[0058]Embodiment 3: detect Mycoplasma pneumoniae IgM monoclonal antibody with described test paper, comprise the following steps: Sample spot is approached on the assembled test paper one end of Mycoplasma pneumoniae IgM monoclonal antibody, after reacting 5min, in the ultraviolet analyzer Observation results. PBS buffer solution and normal human blood were used as blank controls.
[0059] Result judgment: under the premise that the C band shows a red fluorescent band, the intensity of the fluorescent band of the T band is visually compared with the blank. The weaker the fluorescence, the lower the concentration of the tested substance in the test solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com