Mutation method for enhancing beta-cyclodextrin production capacity of beta-cyclodextrin glycosyltransferase
A glucosyl and cyclodextrin technology, which is applied in the fields of genetic engineering and enzyme engineering, can solve the problems of inconvenient separation and purification of a single type of cyclodextrin product, and achieve the effect of improving specificity and facilitating industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: This example illustrates the preparation of mutants A31R, A31P and A31T.
[0029] (1) Site-directed mutation
[0030] Using rapid PCR technology, site-directed mutagenesis was carried out with the expression vector pST / cgt containing the wild CGTase gene as a template.
[0031] Primers for site-directed mutagenesis introducing the Arg31 codon:
[0032] Forward primer: 5'-GACGGCAATCCC CGC AACAATCC-3', the underline is the mutated base,
[0033] Reverse primer: 5'-GGATTGTT GCG GGGATTGCCGTC-3', the underline is the mutant base;
[0034] Primers for site-directed mutagenesis introducing the Pro31 codon:
[0035] Forward primer: 5'-GACGGCAATCCC CCC AACAATCC-3', the underline is the mutated base,
[0036] Reverse primer: 5'-GGATTGTT GGG GGGATTGCCGTC-3', the underline is the mutant base;
[0037] Primers for site-directed mutagenesis introducing the Thr31 codon:
[0038] Forward primer: 5'-GACGGCAATCCC ACC AACAATCC-3', the underline is the mutated bas...
Embodiment 2
[0046] Example 2: This example illustrates an enzyme activity assay.
[0047] (1) Determination of enzyme activity
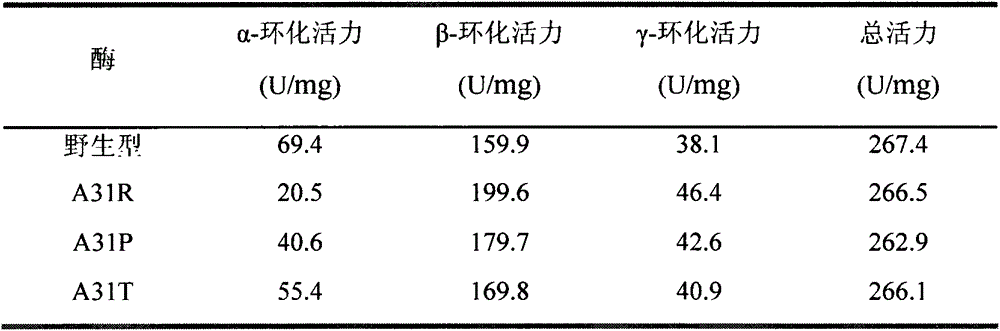
[0048] Determination of α-cyclization activity: Take 0.1 mL of appropriately diluted enzyme solution and add it to a test tube containing 0.9 mL of 1% (w / v) soluble starch solution prepared in advance with 50 mM phosphate buffer (pH 6.0). After reacting at 50°C for 10min, add 1.0mL of 1.0N hydrochloric acid to stop the reaction, then add 1.0mL of 0.1mM methyl orange solution prepared with 50mM phosphate buffer solution and incubate at 20°C for 15min, and measure the absorbance at 505nm. Using the inactivated enzyme as a blank, the content of α-cyclodextrin was determined corresponding to the α-cyclodextrin standard curve. One enzyme activity unit is defined as the amount of enzyme required to generate 1 μmol of cyclodextrin per minute under the above conditions.
[0049] Determination of β-cyclization activity: Take 0.1 mL of appropriately diluted enzyme solut...
Embodiment 3
[0055] Example 3: This example illustrates the use of HPLC to analyze the amount of cyclodextrin produced
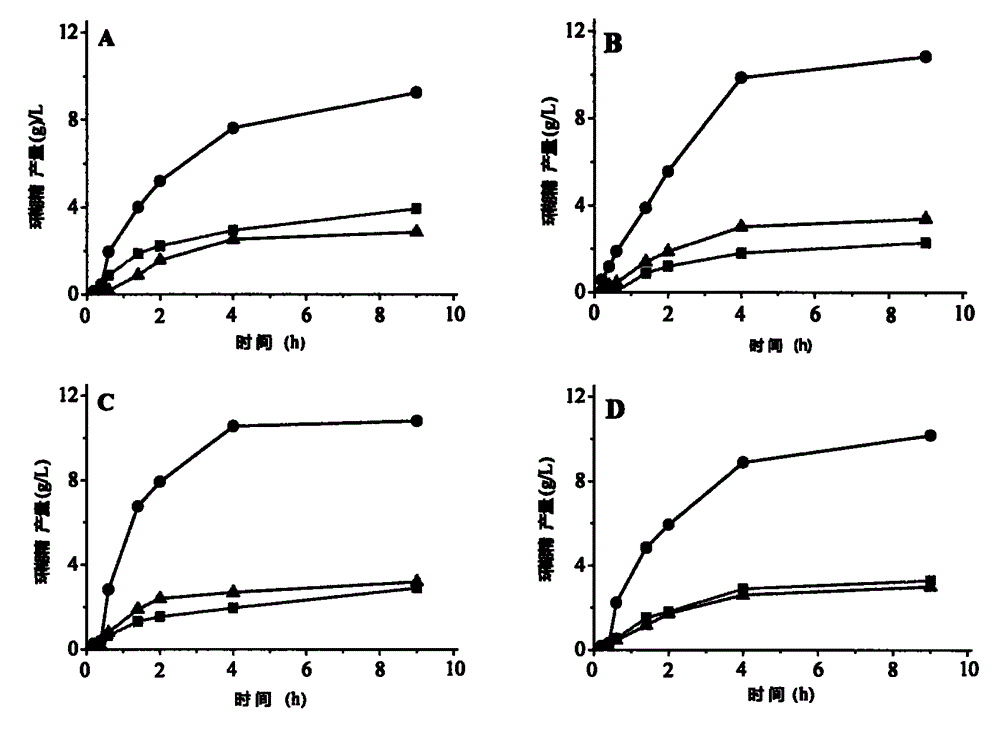
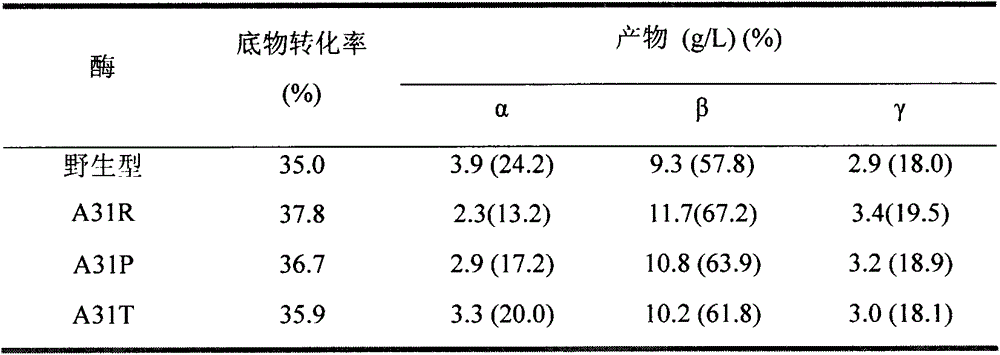
[0056] To prepare 5% (wet basis, water content 8%, w / v) maltodextrin (DE3) solution as substrate, 5g maltodextrin (DE3) was dissolved in 90mL sodium phosphate buffer (pH6.0), fixed Make up to 100mL, boil in boiling water for 30min. Add a certain amount of wild CGTase, mutants A31R, A31P, and A31T to make the enzyme activity in the reaction system 1U / mL, place it at 50°C for 9 hours, sample 600 μL at intervals, boil the enzyme for 10 minutes, centrifuge at 12000rpm for 10 minutes, and take Add 5 μL of glucoamylase (70 U / mL) to 500 μL of supernatant, saccharify at 30°C for 1 hour, boil for 10 minutes to inactivate, centrifuge at 12,000 rpm for 30 minutes, and take 20 μL of the supernatant for HPLC analysis after filtering through a 0.45 μm ultrafiltration membrane.
[0057] HPLC determination conditions are: Waters600 high performance liquid chromatograph (with differenti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com