Method for separating guaiacol glyceryl ether enantiomer through simulated moving bed chromatography technology
A technology of guaiacol glyceryl ether and simulated moving bed, which is applied in the field of resolution of chiral compounds, can solve problems such as the resolution of guaiacol glycerin ether that have not been seen before, and achieve a pollution-free production mode, high degree of automation, The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
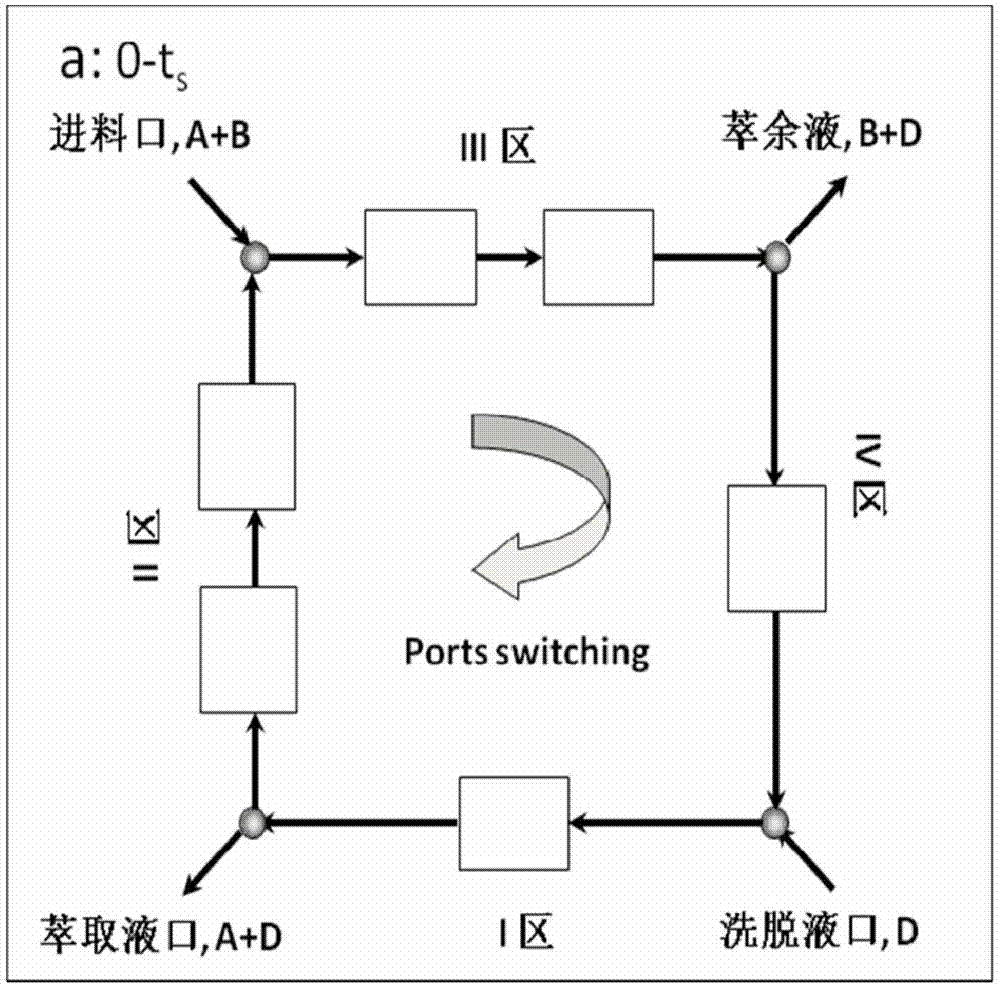
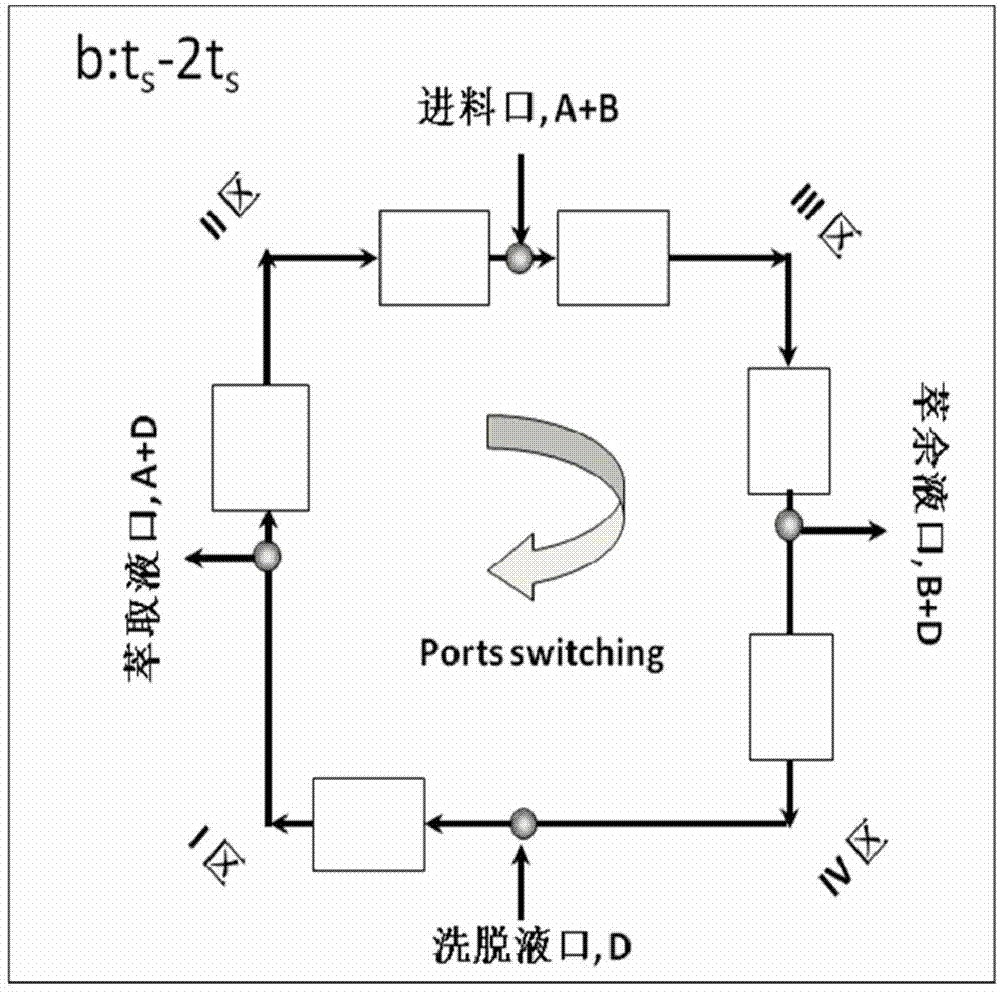
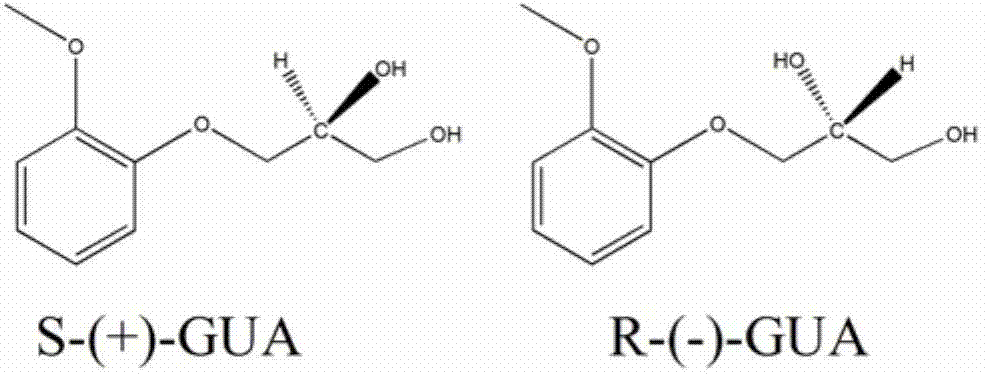
[0023] 1. A simulated moving bed chromatography system is adopted, which includes a feed pump, an eluent pump, an extraction pump, a raffinate pump, a circulation pump, a solenoid valve, a detector, a chromatographic column and a computer automatic control system. The schematic diagram of the principle is as follows Figure 1a and 1b shown. The sample solution and the eluent enter the system from the feed liquid inlet and the eluent inlet respectively, and the products S-(+)-guaiacol and R-(-)-guaiacol respectively come from the extraction Liquid and raffinate are collected from two outlets. After one switching cycle, two inlets: sample liquid and eluent, two outlets: extract liquid and raffinate, switch to the next chromatographic column position along the direction of mobile phase at the same time, such as Figure 1b shown.
[0024] 2. Column packing and mobile phase selection
[0025] The filler is cellulose-tris(3,5)-dimethylphenylcarbamate coated on silica gel, prepare...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com