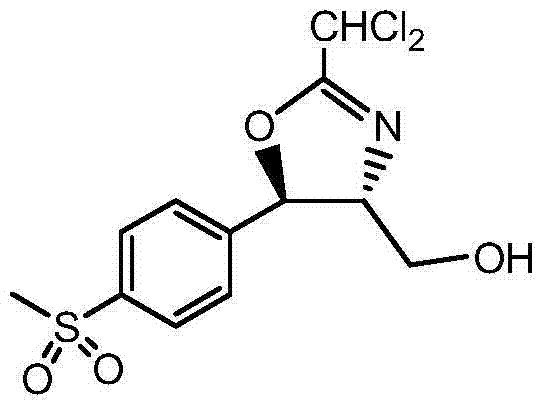
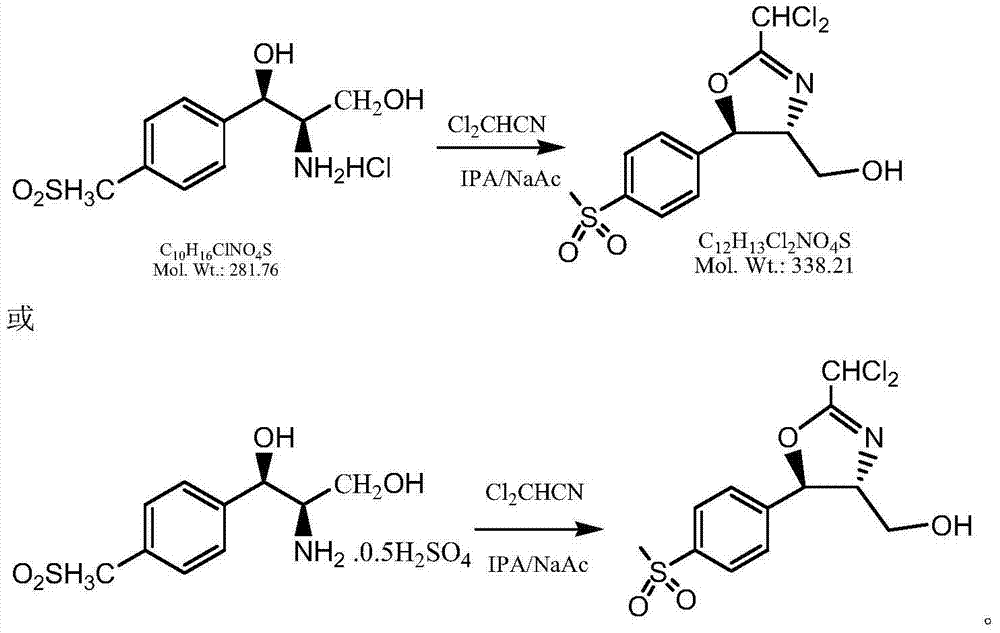
Synthetic method of florfenicol intermediate cyclic product
A technology of florfenicol and synthetic method, which is applied in the field of synthesis of florfenicol intermediate cyclic compounds, can solve problems such as difficult operation, difficult post-processing, long time, etc., and achieves simplified operation process, shortened production cycle, The effect of improving productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
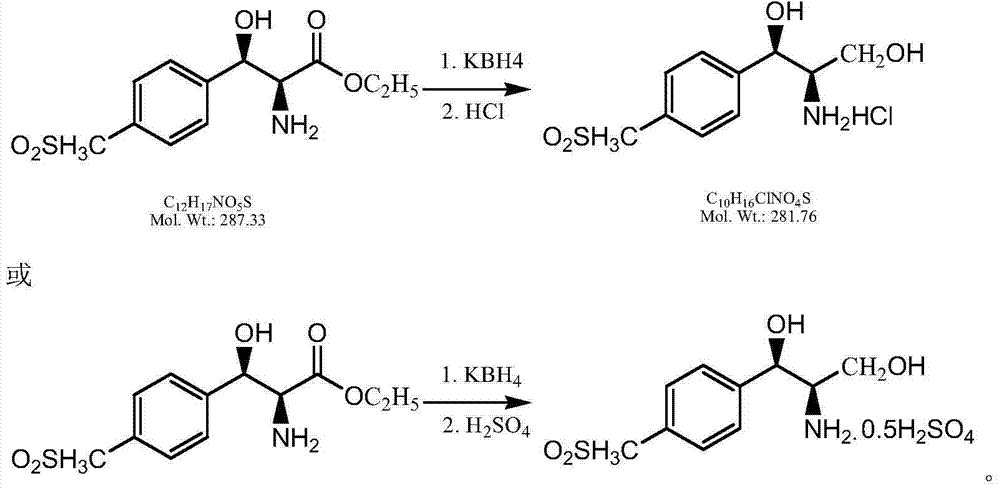
[0035] Add 300ml of methanol, 5g of anhydrous calcium chloride and 50g of D-p-thymphenylphenylserine ethyl ester into a 500ml three-neck flask, stir evenly, add 12g of potassium borohydride in four times, and add 3g of potassium borohydride every half an hour , Control the internal temperature of the reaction not to exceed 40°C. After the addition, keep the reaction at 45°C for 4 hours. Adjust the pH of the reaction solution to 2.5 with concentrated hydrochloric acid, evaporate about half of the methanol under reduced pressure, then lower the temperature to below 10°C, filter with suction, wash the filter cake with 20ml of cold methanol, and dry to obtain 47.1g of thiamphenicolamine hydrochloride, white Powder, yield: 96.04%, purity: 99%.
Embodiment 2
[0037] Add 300ml of methanol, 5g of anhydrous calcium chloride and 50g of D-p-thymphenylphenylserine ethyl ester into a 500ml three-neck flask, stir evenly, add 12g of potassium borohydride in four times, and add 3g of potassium borohydride every half an hour , Control the internal temperature of the reaction not to exceed 40°C. After the addition, keep the reaction at 45°C for 3.5 hours. Adjust the pH of the reaction solution to 2.2 with concentrated sulfuric acid, distill off about half of the methanol under reduced pressure, then cool down to below 10°C, filter with suction, wash the filter cake with 20ml of cold methanol, and dry to obtain 50.2g of thiamphenicolamine sulfate as a white powder , yield: 98.24%, purity: 99%.
Embodiment 3
[0039] Add 300ml of ethanol, 10g of anhydrous calcium chloride and 50g of D-p-thymphenylphenylserine ethyl ester into a 500ml three-neck flask, stir evenly, add 12g of potassium borohydride in four times, and add 3g of potassium borohydride every half an hour , Control the internal temperature of the reaction not to exceed 40°C. After the addition, keep the reaction at 50°C for 3 hours. Adjust the pH of the reaction solution to 2.6 with concentrated sulfuric acid, evaporate about half of the ethanol under reduced pressure, then cool down to below 10°C, filter with suction, wash the filter cake with 20ml of cold ethanol, and dry to obtain 50.3g of thiamphenicolamine sulfate as a white powder , yield: 98.22%, purity: 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com