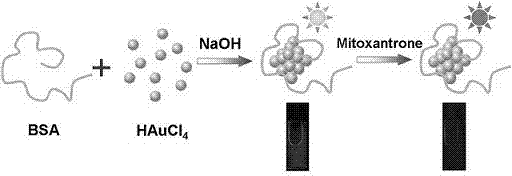
Method for detecting mitoxantrone based on luminous gold nanocluster
A technology of mitoxantrone and gold nanoclusters is applied in the field of mitoxantrone drug detection to achieve the effects of high sensitivity, rapid detection and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Synthesis of fluorescent nano-gold clusters using BSA as a template: The synthesis of fluorescent nano-gold clusters is based on the method reported in the literature [see: Xie J., Zheng Y., Ying J. Y. J. Am. Chem. Soc. , 2009, 131, 888-889.], made some improvements, the process is as follows: First, 1g of tetrachloroauric acid was dissolved in 100 mL of double distilled water to form a 10 mg / mL stock solution. Then, take 4.1 mL of the newly prepared solution and add it to 10 mL of the 50 mg / mL bovine serum albumin solution at a reaction temperature of 37 ? C. During the period, stir vigorously to make it evenly mixed. After 2 minutes, add 1 mL of 1.0 mol / L NaOH solution, continue to stir for 12 hours, keep the temperature at 37 ? C. After the improvement, the synthesis time was shortened from three days to 12 hours. The prepared gold nanocluster probe has stable fluorescence characteristics, with a maximum emission peak at a wavelength of 619 nm and an excitation wavel...
Embodiment 2
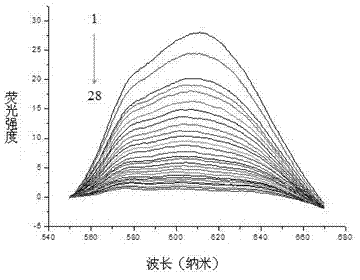
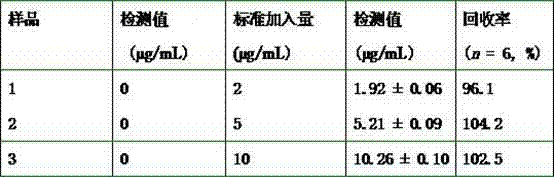
[0022] Use the prepared fluorescent gold nanocluster probe to detect the anticancer drug daunorubicin. Specific steps: Take 300 μL of the prepared gold nanocluster sample, add it to a centrifuge tube, and then add a series of concentrations of daunorubicin drug working solution , To detect the effects of different concentrations of daunorubicin on the fluorescence signal of gold nanocluster probes (final concentrations are 0, 2, 4, 10, 20, 50, 70, 100, 110, 120, 130, 140 μg / mL ). Dilute with double distilled water to a final volume of 3 mL, mix well, and measure. Under the excitation of 469 nm wavelength light, the fluorescence spectrum of gold nanoclusters was tested, the slit width of the instrument was set to 5 nm, and the fluorescence intensity of the emission peak at the wavelength of 619 nm was detected. Test the fluorescence signal of the sample after adding the drug, and record the fluorescence intensity at the position of the maximum emission peak, expressed as: Δ F ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Slit width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com