Synthesis and crystal form-transforming method of taltirelin
A technique for the synthesis of tatirelin, which is applied in the direction of peptides, etc., can solve the problems of difficult pharmaceutical β crystal form, easy racemization of the product, and low yield, so as to ensure safety, simple process, and easy large-scale production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
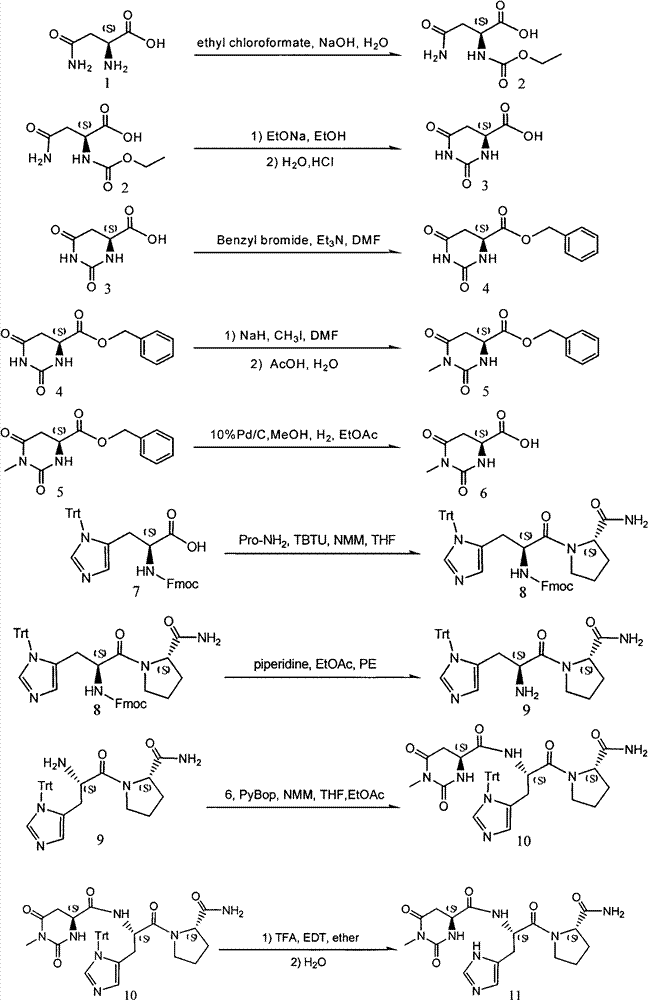
[0030] Example 1 Preparation of S-1-methyl-L-4,5-dihydroorotic acid (compound 6)
[0031] Dissolve 465g of sodium hydroxide (22.2mol) in 5.5L of water, cool down to 5°C, add 1500g of L-asparagine (11.3mol) while stirring, and maintain the temperature at 0-5°C. 141.8 g (13 mol) of ethyl chloroformate was added dropwise, keeping the temperature below 15°C. After the dropwise addition, adjust the pH to 9 with 4N sodium hydroxide solution, react at about 25°C for 4-5 hours, and monitor the reaction by TLC (chloroform:methanol:glacial acetic acid (V:V:V)=20:1:0.5) process. After the reaction was complete, the reaction solution was extracted twice with 2.5 L of dichloromethane, and the organic phase was discarded. Use 6N hydrochloric acid to adjust the pH of the aqueous phase to 2-3, cool and crystallize overnight. Suction filtration, washing the crystal once with cold water, and vacuum drying at 50°C (until the water content is less than 0.05%) gave 1850 g of white solid (Compou...
Embodiment 2
[0036] The preparation of embodiment 2 tatirelin (compound 11)
[0037] 2.1 Fmoc-His(Trt)-Pro-NH 2 Synthesis
[0038] 4465g Fmoc-His (Trt) -OH (7.2mol) and 910g Pro-NH 2 (7.9mol) was dissolved in 22L tetrahydrofuran and 5L water, then 1.18L N-methylmorpholine (10.8mol) and 2775g TBTU (8.64mol) were added, and the reaction was stirred at 25-30°C for 2-4 hours. TLC monitors the reaction process (methanol: dichloromethane = 1: 8), after the reaction is complete, adjust the pH to about 7 with a small amount of glacial acetic acid, evaporate the organic solvent under reduced pressure, add 20L ethyl acetate, separate the organic phase, and use Wash the organic phase with 7.5 L each of 5% citric acid, 3% sodium bicarbonate, and saturated brine once, and dry the organic phase with anhydrous magnesium sulfate for 2 hours. Filter off the desiccant, slowly add 140L of petroleum ether to the filtrate under stirring, and stir and crystallize at 25°C for 2 hours. After suction filtration,...
Embodiment 3
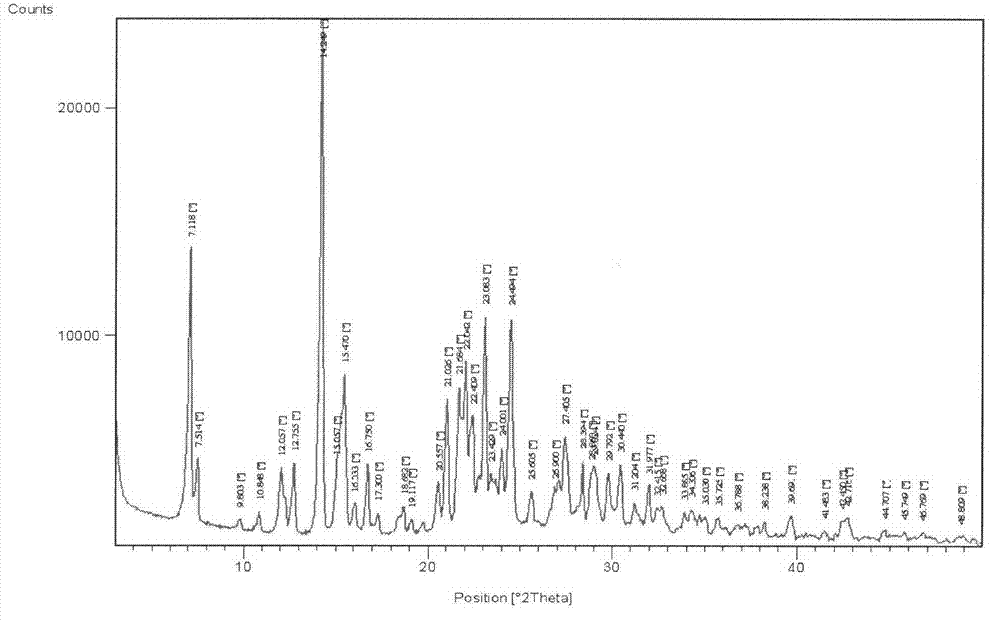
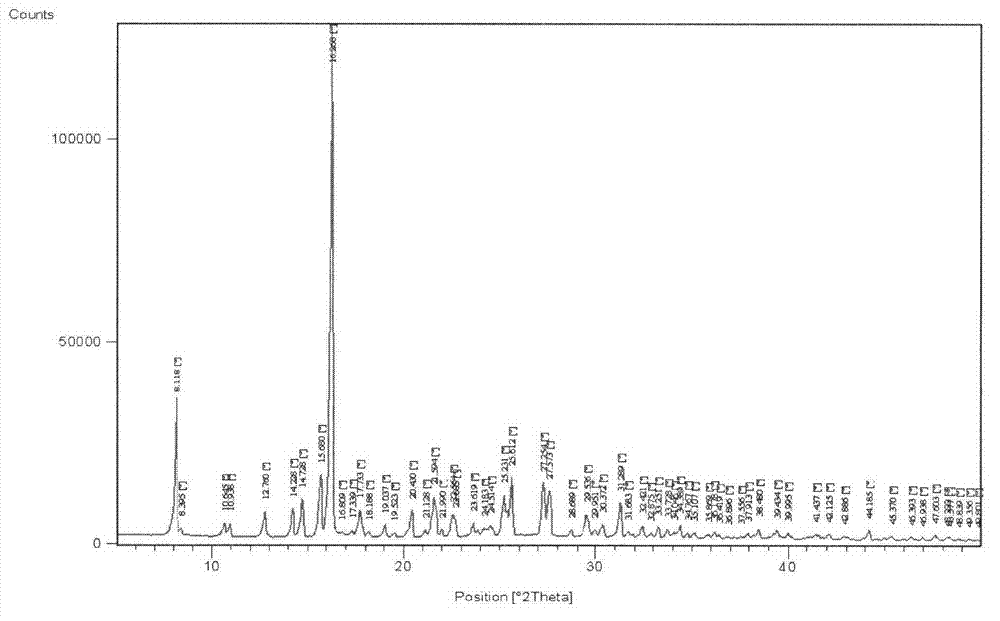
[0044] Embodiment 3 The crystallization and conversion crystal form of tatirelin
[0045] Purify and desalt the white trifluoroacetate crude product of 940g tatirelin (compound 11) by high performance liquid chromatography, obtain white solid 626g after freeze-drying, purification yield 83%, ESI-MS (M+H + ): 406.63 (molecular weight: 405.41).
[0046] Add 620g of lyophilized tatirelin into 1240mL of water, stir and heat to 35-40°C to dissolve, adjust the pH to 9 with saturated aqueous sodium carbonate solution, slowly cool to 5-10°C to crystallize, suction filter, and dry to obtain 582g of α Crystal (C 17 h 23 N 7 o 5 .4H 2 O). The HPLC purity is 99.2%, the melting point is 65-67°C, and the water content is 13%.
[0047] Add 500g of tatirelin α crystals into 1000mL of water, stir and dissolve completely at 35-40°C, then slowly add 100mL of ethanol, slowly cool down to 5-10°C to crystallize, filter with suction, and dry to obtain 466g of β-crystals. HPLC purity 99.6%, m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com