Method for preparing oxygen by electrolyzing carbon dioxide
A carbon dioxide and oxygen technology, applied in the field of electrochemistry, can solve the problems of unsatisfactory catalyst activity and selectivity, high reaction energy consumption, low photoelectric catalytic conversion efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
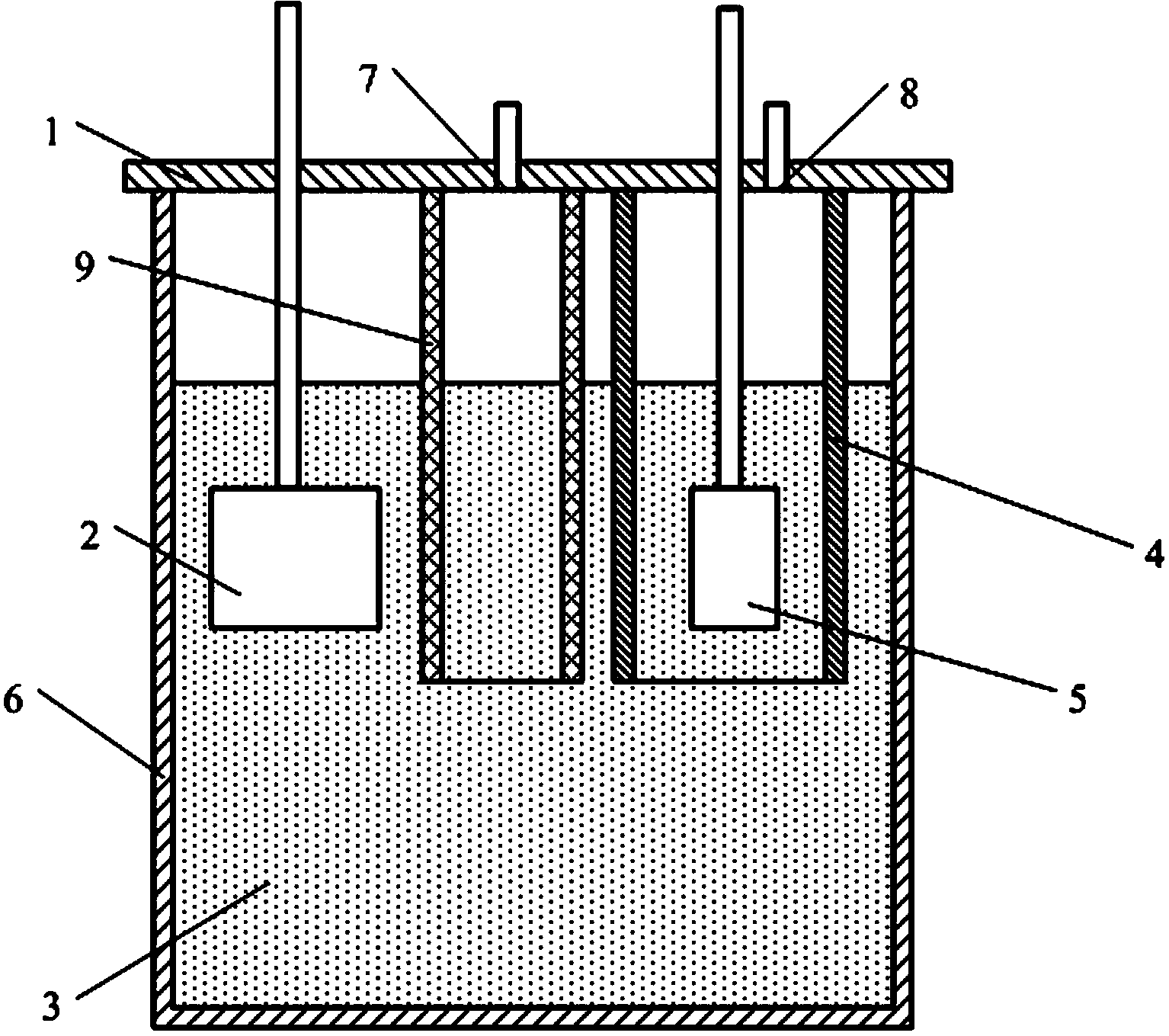
[0024] A device that electrolyzes carbon dioxide to produce oxygen, such as figure 1 As shown, the graphite crucible 6 is included, the top of the graphite crucible 6 is provided with a nickel crucible cover 1, and the nickel crucible 1 cover is provided with CO 2 Inlet 7 and oxygen outlet 8, also be provided with CO on the bottom surface of nickel crucible lid 1 2 Channel 9 and anode cover 4, CO 2 Import 7 with CO 2 The channel communicates with 9, the oxygen outlet 8 communicates with the anode cover 4; the anode 5 and the cathode 2 are inserted into the graphite crucible 6 and the anode 5 is inserted into the anode cover 4;
[0025] Combine anhydrous LiF and Li 2 CO 3 Mix evenly at a molar ratio of 0.5:1 to obtain a mixed material;
[0026] Add additives to the mixed material and mix uniformly to make electrolyte molten salt; the additive accounts for 2% of the total weight of the electrolyte molten salt; the additive is LiCl;
[0027] Using the above device, the grap...
Embodiment 2
[0030] The device for preparing oxygen by electrolysis of carbon dioxide is the same as in Example 1;
[0031] Combine anhydrous LiF and Li 2 CO 3 Mix evenly at a molar ratio of 1.5:1 to obtain a mixed material;
[0032] Add additives to the mixed material and mix uniformly to make electrolyte molten salt; the additive accounts for 15% of the total weight of the electrolyte molten salt; the additive is NaF;
[0033] Using the above device, the graphite crucible is used as the electrolytic cell, the electrolyte molten salt is placed in the electrolytic cell, heated to 690~700°C, and the CO 2 Cylinders and CO 2 Inlet connectivity, then through the CO 2 The channel feeds CO into the electrolyte molten salt 2 gas, and Ni electrode is used as cathode, Fe-Ni alloy electrode is used as anode, and the electrolyte molten salt is energized for electrolysis; the current density is controlled at 0.1A / cm during electrolysis 2 , the incoming CO 2 The pressure of the gas is 0.1~0.12MP...
Embodiment 3
[0036] The device for preparing oxygen by electrolysis of carbon dioxide is the same as in Example 1;
[0037] Combine anhydrous LiF and Li 2 CO 3 Mix evenly at a molar ratio of 1:1 to obtain a mixed material;
[0038] Add additives to the mixed material and mix uniformly to make electrolyte molten salt; the additive accounts for 3% of the total weight of the electrolyte molten salt; the additive is KF;
[0039] Using the above device, the graphite crucible is used as the electrolytic cell, the electrolyte molten salt is placed in the electrolytic cell, heated to 690~700°C, and the CO 2 Cylinders and CO 2 Inlet connectivity, then through the CO 2 The channel feeds CO into the electrolyte molten salt 2 Gas, and use Ni electrode as cathode, using Fe-Ni-Al 2 o 3 The alloy electrode is the anode, and the electrolyte molten salt is energized for electrolysis; the current density is controlled at 0.2A / cm during electrolysis 2 , the incoming CO 2 The pressure of the gas is 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com