Detection method of fluoroquinolone drug residues based on luciferase labelled engineered bacterium
A technology of fluoroquinolones and luciferase, which is applied in the field of veterinary drug residue analysis and genetic engineering, can solve the problems of insufficient detection range of tissue samples, and achieve the effects of wide application range, simple processing method, good accuracy and precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Construction of luciferase-labeled genetically engineered bacteria
[0030] Pick a small amount of Escherichia coli K12 (purchased from China. Wuhan. Wuhan University China Type Culture Collection Center, its preservation number is CCTCC NO: AB200068) freeze-dried powder, inoculate into 10mL LB broth medium, 37°C, 250rpm Shake overnight in a constant temperature shaker. Pick a small amount of bacterial solution to streak and inoculate on LB agar plate, overnight at 37°C. Prepare Escherichia coli K12 into competent cells [The method of bacterial competent cells is a common method, see: J. Sambrook, EF Fritsch, T Maniartis, translated by Huang Peitang, Wang Jiaxi, etc., Molecular Cloning Experiment Guidelines (Third Edition), Beijing, Science Press, 2002 Edition], cryopreserved at -70°C.
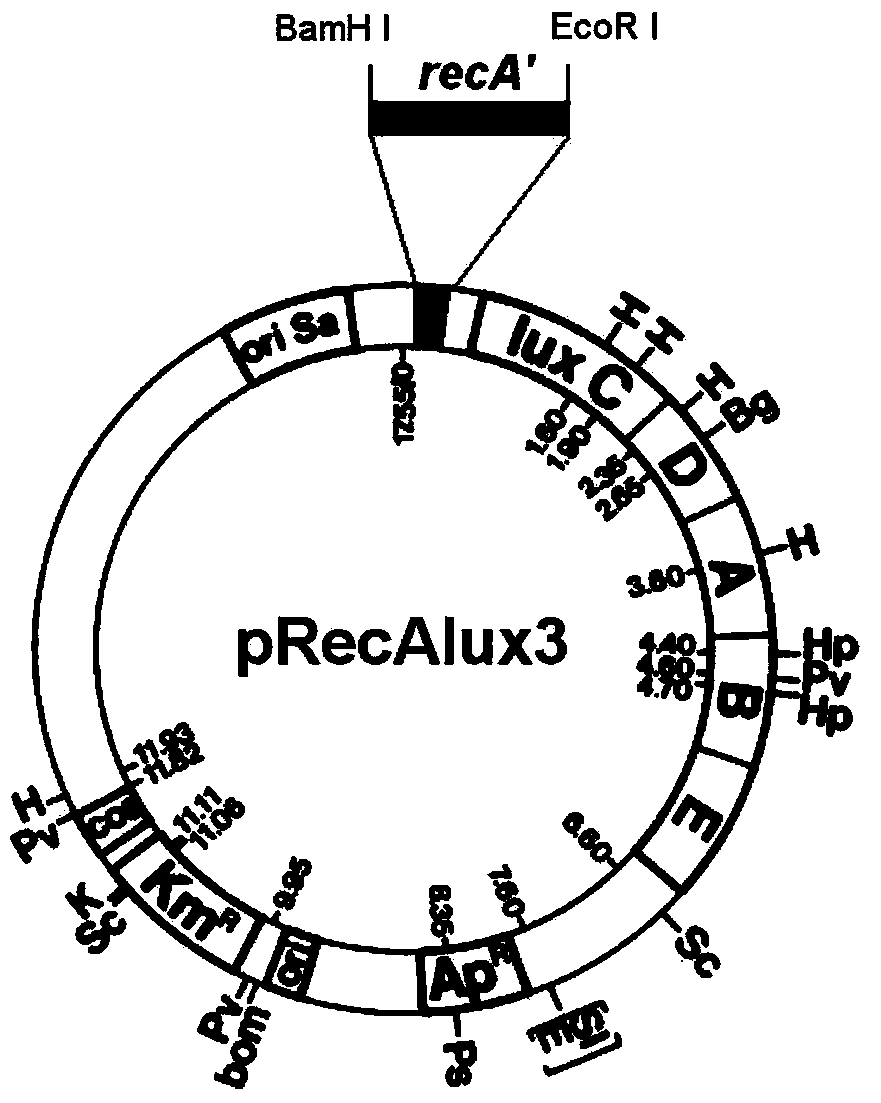
[0031]The plasmid pRecAlux3 (gifted by Professor Amy Cheng Vollmer, Department of Biology, Swarthmore College, USA, and preserved in the National Laboratory of Veterinary Dr...
Embodiment 2
[0032] Example 2 Establishment of Fluoroquinolones Detection Method
[0033] 2.1 Determination of the optimal working concentration of bacterial solution
[0034] Escherichia coli pK12 was cultured in LB liquid medium (containing 25 μg / mL kanamycin) at 26°C and 250 rpm until OD 600 At 0.7 and 1, the sensitivity of Escherichia coli pK12 to enrofloxacin was detected, and the incubation time was 45min; the results showed that the higher the concentration of the bacterial solution in the logarithmic growth period, the higher the sensitivity, so the OD 600 When =1, the bacterial liquid is the working bacterial liquid.
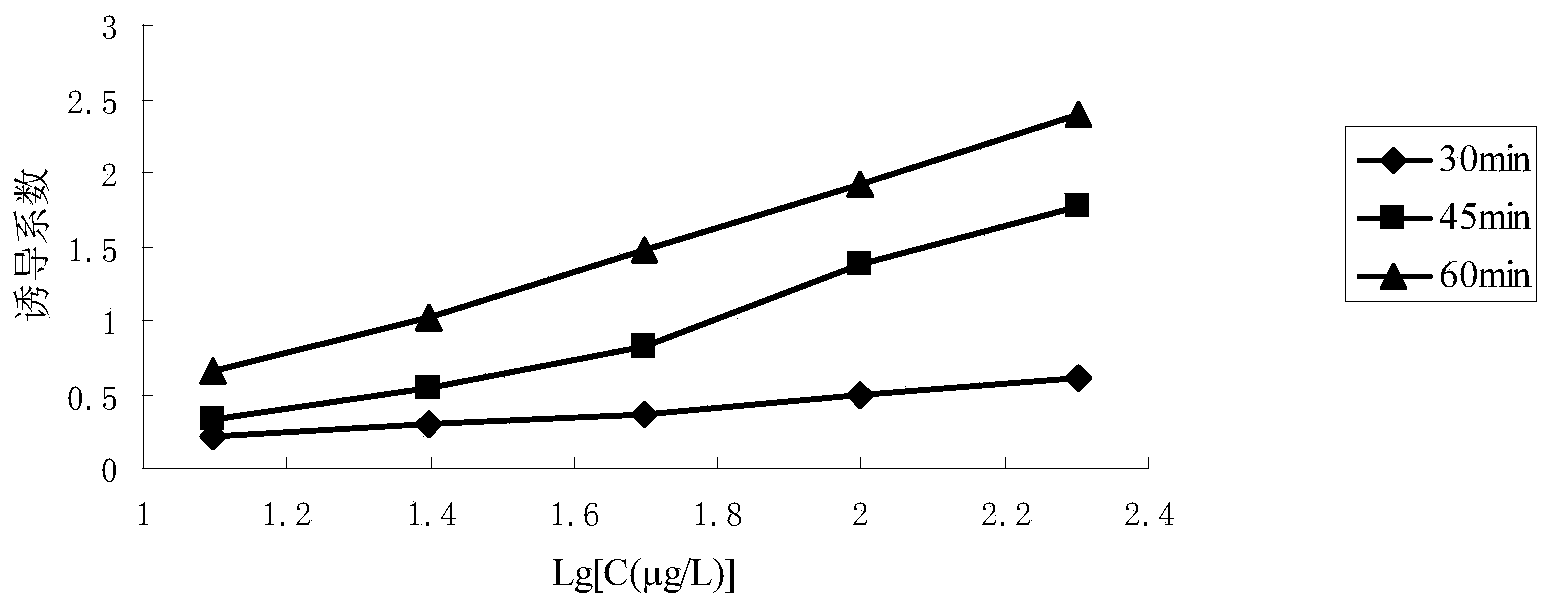
[0035] 2.2 Determination of the optimal incubation time of bacteria solution and drug
[0036] Escherichia coli pK12 was cultured in 10 mL LB liquid medium (containing 25 μg / mL kanamycin) to OD 600 =1, add a series of enrofloxacin (0.025 μg / mL, 0.050 μg / mL, 0.100 μg / mL, 0.200 μg / mL, 0.400 μg / mL, 0.800 μg / mL), respectively Each 50 μL was co-incubated for a certa...
Embodiment 3
[0047] Embodiment 3 The implementation procedure of the method that the present invention establishes
[0048] 3.1 Preparation of reagents
[0049] The preparation method of phosphate buffer solution is: weigh 0.523g KH 2 PO 4 and 16.73g K 2 HPO 4 Dissolve in 800mL distilled water, adjust the pH value to 8.0, dilute to 1L, sterilize under high-pressure steam at 121°C for 20min, and set aside.
[0050] 3.2 Pretreatment of tissue samples
[0051] The pretreatment method of muscle and viscera samples: take muscle, liver, kidney and fish samples of beef, chicken and pig respectively, remove fascia, cut into pieces, and homogenize for 5 minutes. Weigh 1.0 g of homogeneous tissue into a centrifuge tube, add 2 mL of sterile pH 8.0 phosphate buffer, vortex and mix for 5 min, let stand at room temperature for 30 min, centrifuge at 12000 g for 10 min, and take the supernatant for testing.
[0052] Pretreatment method of milk samples: Fresh milk samples can be loaded directly for d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com