Synthetic method of musk tibetene
A Tibetan musk and synthetic method technology, applied in the field of chemical industry, can solve the problems of complex process, low yield, long process, etc., and achieve the effects of low requirements on reaction conditions, simple and efficient synthetic method, and low environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
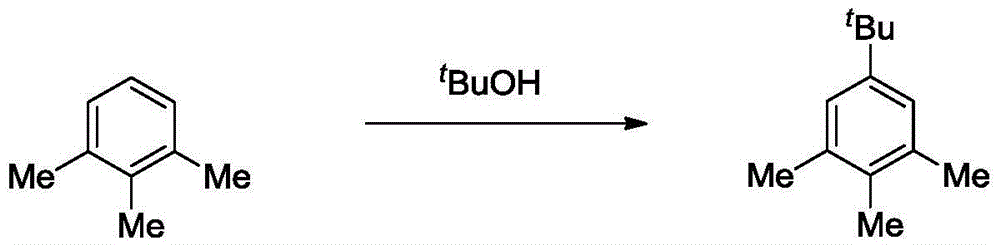
[0018] Embodiment 1: the synthetic method of 5-tert-butylthylene
[0019]
[0020] Add three-necked benzene (1.2g, 10mmol), anhydrous ferric chloride (0.1g, 0.6mmol) and dichloromethane (15mL) in sequence in the three-necked flask, and slowly add tert-butanol (0.5mL, 5mmol) di Chloromethane solution (10 mL), keep the temperature below 80°C during the dropwise addition. After the dropwise addition, it was heated to 80° C. to continue the reaction for 2 hours. After cooling, it was quenched with ice water, separated, and the aqueous phase was extracted three times with dichloromethane (15mL). The combined organic phase was washed with saturated brine and anhydrous Na 2 SO 4 dry. Filter and remove solvent under reduced pressure. The residue was distilled under reduced pressure, and the 85-88°C fraction (1.33kPa) was collected to obtain 5-tert-butylthylene (1.26g, 72.0%).
Embodiment 2
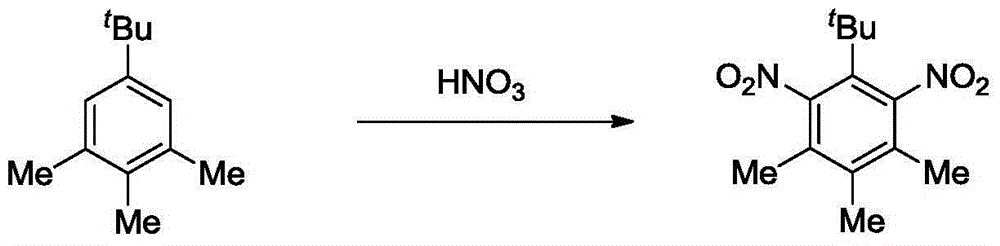
[0021] Example 2: Synthesis of Tibetan musk from 5-tert-butylthylene
[0022]
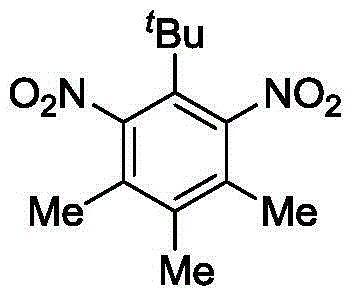
[0023] Cuprous chloride (0.1g, 1.0mmol), anhydrous acetic acid (1.0mL), 5-tert-butylthylene (1.76g, 10.0mmol) and carbon tetrachloride (30mL) were added to the flask, and the Fuming nitric acid (1.2 mL) was slowly added dropwise, and after 2 hours of reaction, the temperature was slowly raised to room temperature and the reaction was continued for 6 hours. After the reaction is completed, dilute with ice water, neutralize, and filter, and the filter cake is washed with water and dried to obtain the crude product of Tibetan musk.
[0024] Characterization data: m.p.134-136°C; 1 H NMR (400MHz, CDCl 3 )δ2.41(6H,s),2.20(3H,s),1.33(9H,s); 13 C NMR (100MHz, CDCl 3 )δ151.2, 134.7, 134.5, 133.6, 29.3, 27.9, 15.9, 14.1; ESI-MS, m / z=267.5 ([M+H] + ).
Embodiment 3
[0025] Embodiment 3: the recrystallization of Tibetan musk synthetic crude product
[0026] The crude product of Tibetan musk (3.0g) was heated to 80°C with ethanol (20mL) as a solvent, filtered while hot after dissolution, slowly cooled to 40-45°C, kept for 1 hour, and filtered. Repeat 2 times to obtain pure Tibetan musk with a purity greater than 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com