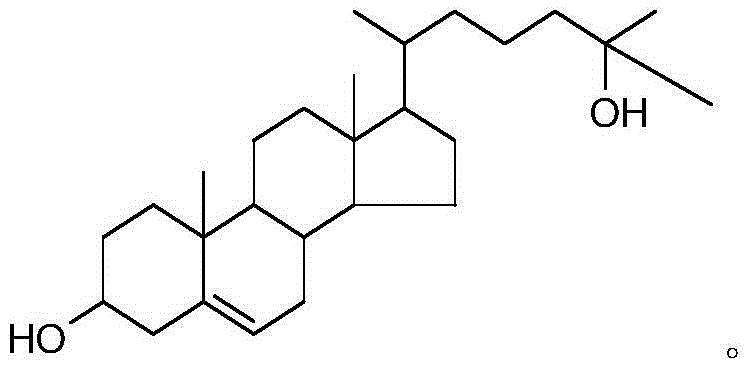
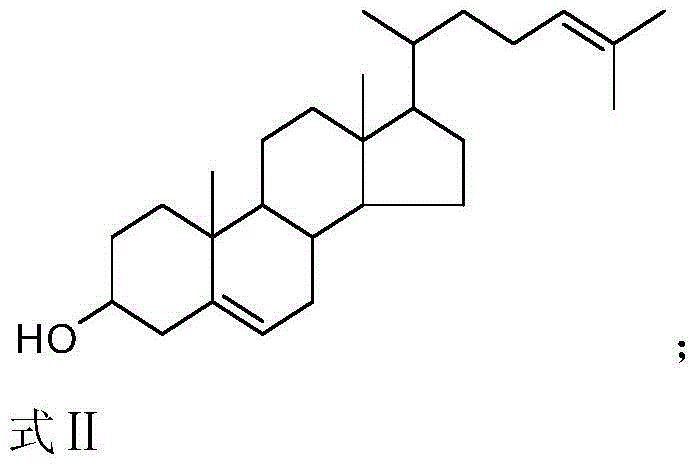
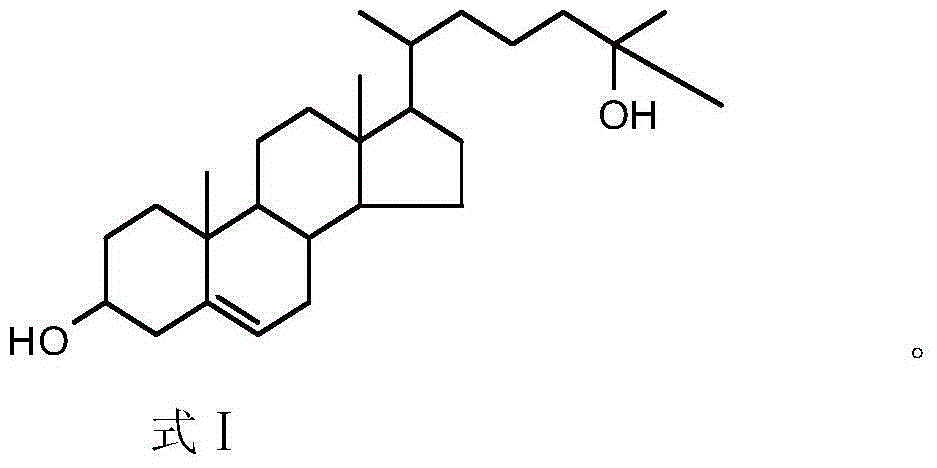
Production method of 25-hydroxycholesterol
A technology of hydroxycholesterol and its production method, which is applied in the direction of steroids and organic chemistry, can solve the problems of poor selectivity and low yield, and achieve the effects of short reaction time, reduced production cost and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, a kind of production method of 25-hydroxycholesterol, carries out following steps successively:
[0037] 1) Add 20g of 24-dehydrocholesterol (about 0.052mol) and 0.2g of 4-dimethylaminopyridine (DMAP) to 150mL of pyridine (also used as a solvent and acid-binding agent), and add 10g (about 0.097mmol) acetic anhydride (dropped in about 30 minutes). The reaction process was monitored by TLC. After the dropwise addition, the reaction was continued at room temperature for 10 h, and the reaction ended.
[0038] The reaction solution was washed with water (the amount of water used was 50ml×2), pickled (using a dilute hydrochloric acid solution with a volume concentration of 5%, the amount used was 50ml×2), and then extracted with dichloromethane (the amount used was 30ml×3). Wash with saturated sodium bicarbonate solution until neutral, dry over anhydrous sodium sulfate (about 5 g) and remove the solvent (ie, dichloromethane) to obtain 19.1 g of acetylated 24-de...
Embodiment 2
[0047] Embodiment 2, a kind of production method of 25-hydroxycholesterol,
[0048] Utilize the hydroxybromination product of embodiment 1 step 2) gained, then carry out following step 3):
[0049] Dissolve 0.523 g of the hydroxy bromide product (1 mmol) in 20 mL of sodium ethoxide (0.27 g, 4 mmol) in ethanol, add 160 mg of Lindella catalyst (10% palladium content, 0.15 mmol of palladium), and the reaction mixture The reaction is carried out at 0°C and 0.1 MPa of hydrogen, and the reaction is stopped when the hydrogen pressure basically no longer drops (the reaction time is about 5 to 6 hours). The reaction results were detected by HPLC.
[0050] The resulting reaction solution was filtered, the filtrate was washed with 5% dilute hydrochloric acid solution and extracted with dichloromethane, the obtained extract was washed with saturated sodium bicarbonate solution and saturated sodium chloride solution in turn, dried with anhydrous sodium sulfate, and rotated under reduced p...
Embodiment 3
[0051] Embodiment 3, a kind of production method of 25-hydroxycholesterol,
[0052] Utilize the hydroxybromination product of embodiment 1 step 2) gained, then carry out following step 3):
[0053] 0.523 g of hydroxybrominated product (1 mmol) was dissolved in 40 mL of glycerol solution, 210 mg of Lindella catalyst (5% palladium content, 0.1 mmol), 0.08 g of sodium hydroxide solid (2 mmol) were added, and the reaction mixture was The reaction is carried out in 0.1MPa hydrogen at 20°C, and the reaction is stopped when the hydrogen pressure basically no longer drops (the reaction time is about 5 to 6 hours). The reaction results were detected by HPLC.
[0054] The resulting reaction solution was filtered, the filtrate was washed with 5% dilute hydrochloric acid solution and extracted with dichloromethane, the obtained extract was washed with saturated sodium bicarbonate solution and saturated sodium chloride solution in turn, dried with anhydrous sodium sulfate, and rotated und...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com