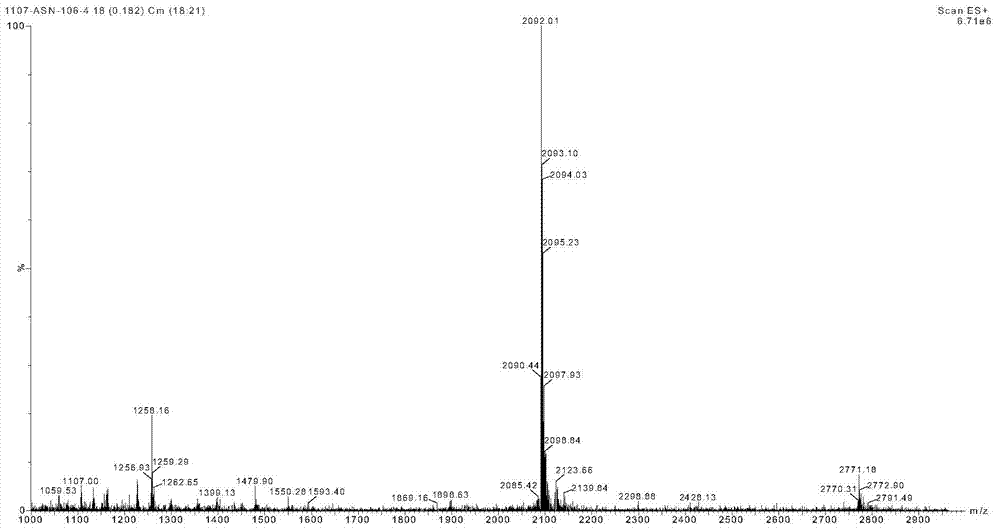
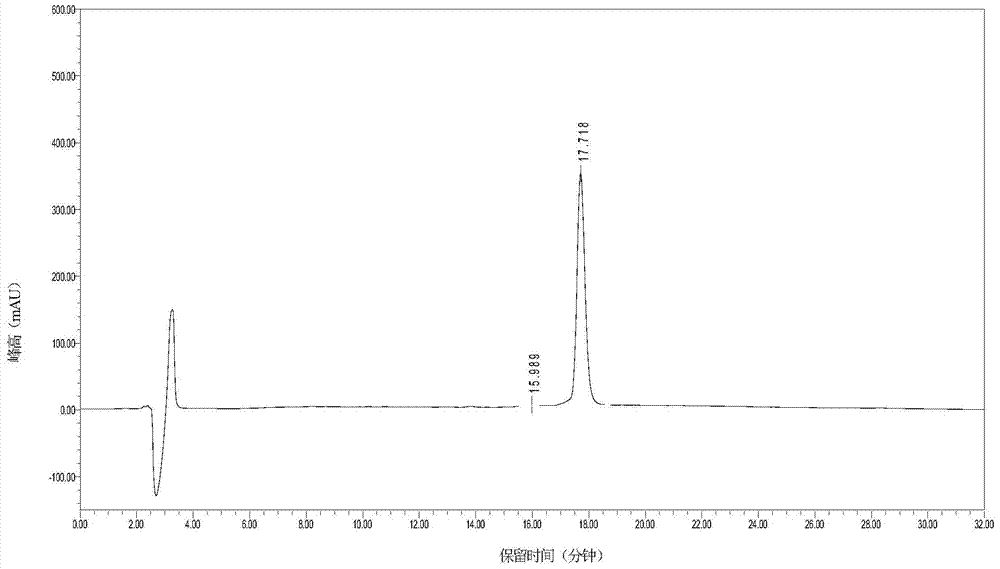
Method for low-cost purification of exenatide
A low-cost exenatide technology, applied in the field of peptide purification, to achieve high yield, cost saving, and environmental protection of the purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1. Sample dissolution
[0028] Dissolve 0.2 g of the crude exenatide obtained by solid-phase synthesis in 5 mL of 20% acetic acid aqueous solution, adjust the pH value to 4.0 with 15% sodium hydroxide aqueous solution, and disperse it ultrasonically. After the solution is completely clarified, Filter through a 0.45 μm membrane filter and collect the filtrate.
[0029] 2. Crude and pure
[0030] The filtrate was crudely purified by ion-exchange high-performance liquid chromatography. The filler was a strong cation-exchange column of SP high-flow rate agarose microspheres (provided by GE Healthcare) with a particle size of 45-165 μm. The column packing volume was 15 mL. Phase A is 0.02mol / L acetic acid-sodium acetate aqueous solution with a pH value of 4.0, mobile phase B is 0.02mol / L acetic acid-sodium acetate aqueous solution with a pH value of 4.0 containing 1mol / L sodium chloride, and the flow rate is 4mL / min , the column temperature is 40°C, and the detection wavel...
Embodiment 2
[0036] 1. Sample dissolution
[0037] Dissolve 1.5 g of the crude exenatide obtained by solid-phase synthesis in 50 mL of 20% acetic acid aqueous solution, adjust the pH value to 4.0 with 15% sodium hydroxide aqueous solution, and disperse ultrasonically until the solution is completely clarified. Filter through a 0.45 μm membrane filter and collect the filtrate.
[0038] 2. Crude and pure
[0039]The filtrate was crudely purified by ion-exchange high-performance liquid chromatography, and the filler was a strong cation exchange column of SP high-flow rate agarose microspheres with a particle size of 50-160 μm (provided by Xi’an Jiaotong University Baosai Biotechnology Co., Ltd.). The filling volume is 150mL, the mobile phase A is 0.02mol / L acetic acid-sodium acetate aqueous solution with a pH value of 4.0, and the mobile phase B is 0.02mol / L acetic acid-sodium acetate with a pH value of 4.0 containing 1mol / L sodium chloride Aqueous solution, the flow rate is 8mL / min, the co...
Embodiment 3
[0045] In the crude purification step 2 of this embodiment, the mobile phase gradient is selected from 0 to 60 minutes A:B from 60:40 to 30:70. The other steps were the same as in Example 2 to obtain exenatide with a purity greater than 98%, and the purification yield was 50%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com