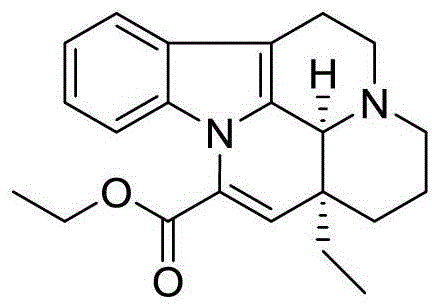
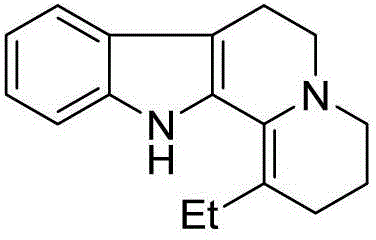
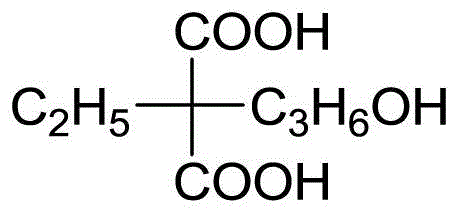Method for preparing vinpocetine intermediate gamma-hydroxypropyl-ethylmalonic acid
A technology of ethylmalonate and diethyl ethylmalonate, which is applied in the field of preparing and synthesizing vinpocetine intermediate γ-hydroxypropyl-ethylmalonate, can solve complex operation, large amount of ethanol, Increase the process cost and other problems, to achieve the effect of simple experimental operation, simplified operation and cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 500mL of anhydrous tetrahydrofuran into a 2L dry four-necked bottle, mechanically stir and add 68g (1.98mol) of 70% sodium hydride at the same time, the reaction solution is gray and turbid. At 10°C, 282 g (1.5 mol) of diethyl 2-ethylmalonate was slowly added dropwise, and the dropwise addition was completed in 2 hours, followed by mechanical stirring for 1 hour. Then, 234 g (1.5 mol) of 1-bromo-3-chloropropane was slowly added dropwise, and the dropwise addition was completed within 2 hours, followed by stirring for 8 hours. 300 mL of water was added to the solution and stirred for 1 hour. Extracted with ethyl acetate and concentrated under reduced pressure to obtain 394 g of light yellow oily product γ-chloropropyl-ethylmalonate diethyl ester, the yield was 99%, and the content was 95.8%.
[0018] Add 394g of diethyl ethylmalonate to a 2L four-neck flask, add 200mL of ethanol, and then add 600mL of a prepared 25% sodium hydroxide aqueous solution. Raise the temp...
Embodiment 2
[0020] Add 400mL of N,N-dimethylformamide (DMF) into a 2L dry four-necked bottle, mechanically stir and add 56g (1.63mol) of 70% sodium hydride at the same time, the reaction solution is gray and turbid. At 40°C, 282 g (1.5 mol) of diethyl 2-ethylmalonate was slowly added dropwise, and the dropwise addition was completed in 2 hours, followed by mechanical stirring for 1 hour. Then, 281 g (1.8 mol) of 1-bromo-3-chloropropane was slowly added dropwise, and the dropwise addition was completed within 2 hours, followed by stirring for 15 hours. Add 300 mL of water to the solution and stir for 1 h. Extracted with ethyl acetate and concentrated under reduced pressure to obtain 389 g of light yellow oil γ-chloropropyl-ethylmalonate diethyl ester, the yield was 98%, and the content was 95.2% (GC).
[0021] Add 389g of diethyl ethylmalonate to a 2L four-neck flask, add 200mL of ethanol, and then add 600mL of a prepared 25% sodium hydroxide aqueous solution. Raise the temperature and r...
Embodiment 3
[0023] Add 500mL of anhydrous tetrahydrofuran into a 2L dry four-necked bottle, mechanically stir and add 60g (1.75mol) of 70% sodium hydride at the same time, the reaction solution is gray and turbid. At 20°C, 282 g (1.5 mol) of diethyl 2-ethylmalonate was slowly added dropwise, and the dropwise addition was completed in 2 hours, followed by mechanical stirring for 1 hour. Then, 240 g (1.54 mol) of 1-bromo-3-chloropropane was slowly added dropwise, and the dropwise addition was completed within 2 hours, followed by stirring for 12 hours. 300 mL of water was added to the solution and stirred for 1 hour. Extracted with ethyl acetate, concentrated under reduced pressure to obtain 395 g of light yellow oily product γ-chloropropyl-ethylmalonate diethyl ester, the yield was 99%, and the content was 96.5% (GC).
[0024] Add 395g of diethyl ethylmalonate to a 2L four-neck flask, add 200mL of ethanol, and then add 600mL of a prepared 25% sodium hydroxide aqueous solution. Raise the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com