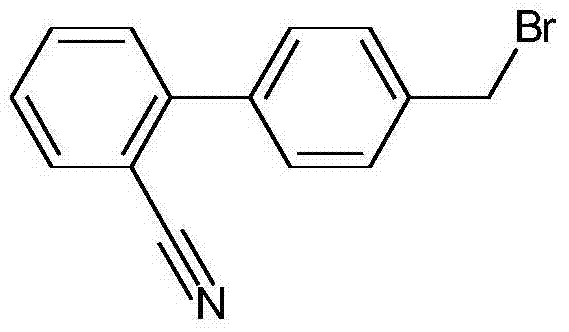
Crystallizing method for preparing high-purity 4-bromomethyl-2-cyanobiphenyl
A technique for bromosartan biphenyl and crude sartan biphenyl, which is applied in the field of crystallization for preparing high-purity bromosartan biphenyl, and can solve the problem that the mass yield is not more than 75%, the purity is lower than 99%, and it is difficult to Obtaining yield and other issues to achieve the effect of reducing side reactions, high yield per pass, and uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
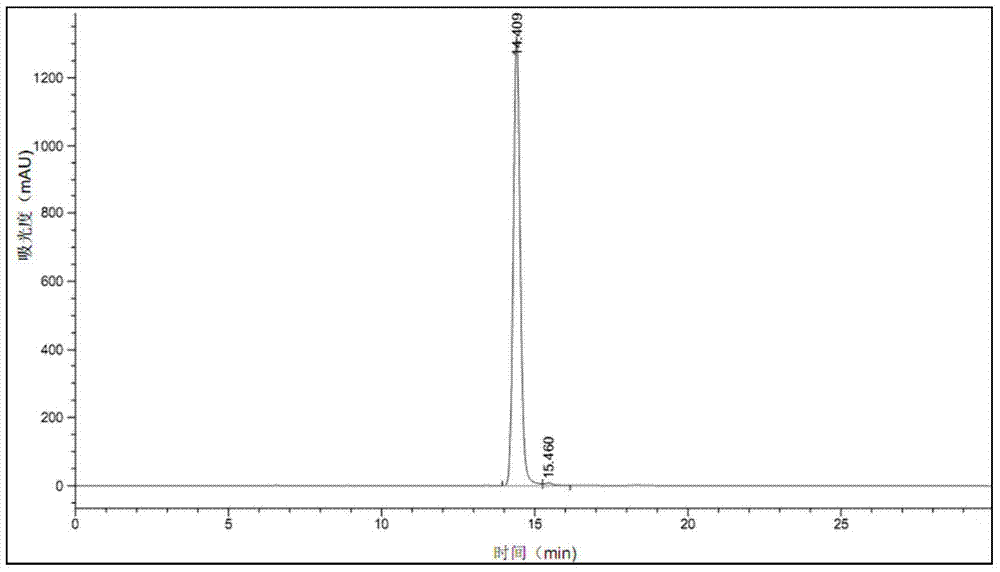
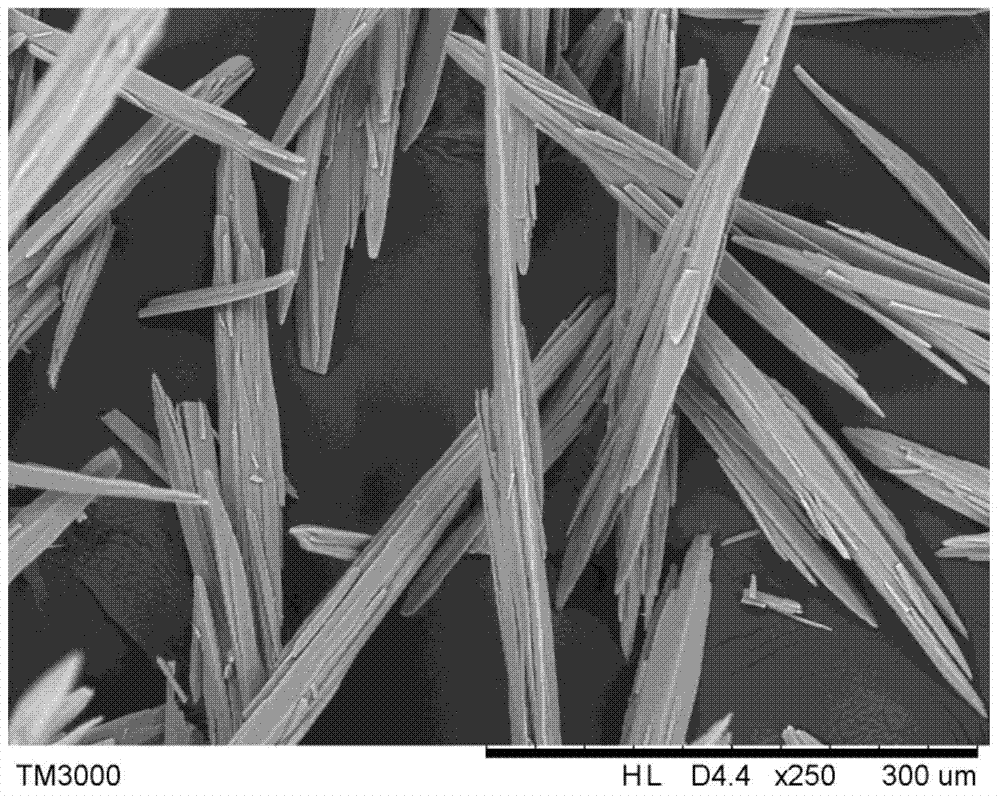
[0027] Add 40.00g of the bromosartan biphenyl crude product with a chromatographic purity of 90% into 100mL of DMF and dissolve it with continuous stirring at 60°C. Cool down to 20°C at a cooling rate of 10°C / hr; add 10mL water and 30mL ethanol mixed eluent at a rate of 16mL / hr after constant temperature for 30min; filter the crystal slurry with suction, wash with water, and dry the crystal at 50°C for 5hr; 34.89 g of bromosartan biphenyl crystals were obtained. The purity of bromosartan biphenyl detected by HPLC was 99.6%, the product quality yield was 96.9%, and the primary particle size was 98 μm.
Embodiment 2
[0029] Add 64.29g of bromosartan biphenyl crude product with a chromatographic purity of 70% to 100mL of tetrahydrofuran, stir and dissolve at 50°C, and connect the reflux tube to the top of the crystallizer to prevent solvent volatilization; carry out the cooling and crystallization process under stirring, at 8°C / hr cooling rate to 10°C; after constant temperature for 60min, add 5mL water and 45mL isopropanol mixed eluent at a rate of 25mL / hr; suction filter the crystal slurry, wash with water, and dry the crystal at 35°C for 6hr; Bromosartan biphenyl crystal 43.35g. The purity of bromosartan biphenyl was detected by HPLC: 99.5%, the product quality yield: 96.34%, and the main particle size was 102 μm.
Embodiment 3
[0031] Add 64.10g of bromosartan biphenyl crude product with a chromatographic purity of 76% to 100mL of toluene, stir and dissolve at 55°C, and connect the reflux tube to the top of the crystallizer to prevent solvent volatilization; carry out cooling and crystallization process under stirring, at 10°C / hr The cooling rate was lowered to 10°C; after a constant temperature of 45min, 100mL of methanol eluent was added at a rate of 30mL / hr; the crystal slurry was filtered with suction, washed with a mixed solution of water and ethanol, and the crystal was dried at 55°C for 7hr; bromosand was obtained 47.40 g of tansbiphenyl crystals. The purity of bromosartan biphenyl was detected by HPLC: 99.7%, the product quality yield: 97.30%, and the main particle size was 95 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com