Solid oxidized glutathione salt and production method thereof
A technology of oxidized glutathione and glutathione salt, applied in chemical instruments and methods, cosmetic preparations, peptides, etc., can solve the problems of high deliquescence, hindering the target reaction, unsuitable for industrial production, etc. The effect of low deliquescence, less restriction and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
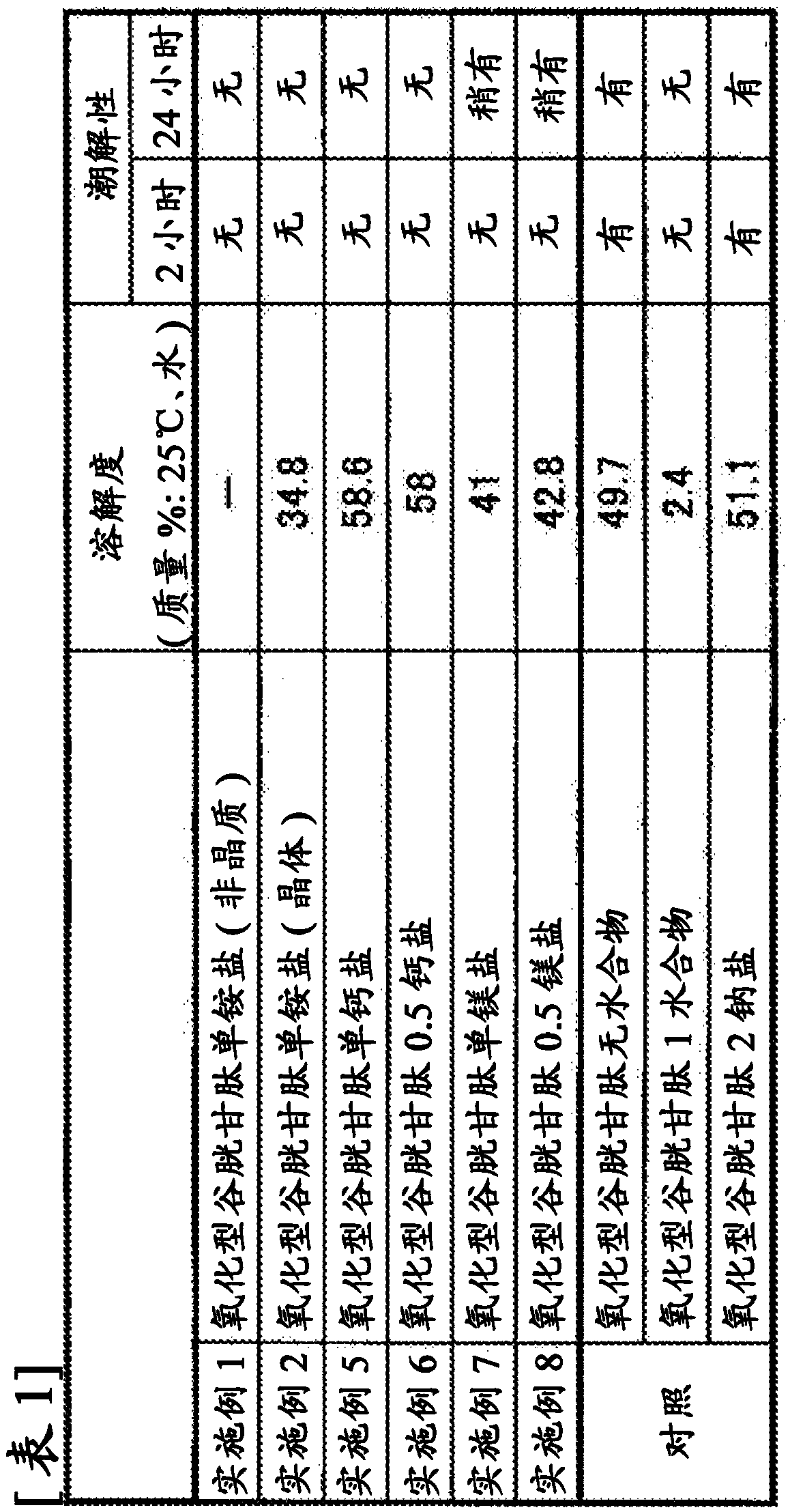
[0097] Dissolve 5 g (8.2 mmol) of oxidized glutathione in 25 g of water to prepare 30 g of an aqueous solution containing oxidized glutathione, and further add 0.56 g (8.2 mmol: molar ratio to glutathione) of 25% ammonia solution =1). While maintaining the temperature of the aqueous solution (pH=3.5) at room temperature (25°C), 90 ml of ethanol (volume ratio to the aqueous solution=3) was added over 30 minutes, and an oily substance was separated from the mixture.
[0098] When the above ethanol-added solution was heated to 30°C, the oil solidified. This state was maintained at a temperature of 30° C. for 15 minutes. After cooling to room temperature, the solid was collected by filtration and dried under reduced pressure to obtain 3.5 g (content: 95% by mass) of oxidized glutathione monoammonium salt.
[0099] Melting point range: 170-176°C (decomposes while melting)
[0100] Amorphous solid (confirmed by X-ray powder diffraction)
Embodiment 2
[0102] Dissolve 3 g (4.9 mmol) of oxidized glutathione in 7 g of water to prepare 10 g of an aqueous solution containing oxidized glutathione, and further add 0.33 g (4.9 mmol: molar ratio to glutathione) of 25% ammonia solution =1). The temperature of this aqueous solution (pH=3.5) was heated to 40° C., and 20 ml of methanol (volume ratio to the aqueous solution=2) was added thereto over 1 hour, to precipitate a solid. After maintaining the temperature at 40° C. for 30 minutes, it was cooled to room temperature, and the solid was recovered by filtration. The solid recovered by filtration was dried under reduced pressure to obtain 2.9 g (content: 99% by mass) of oxidized glutathione monoammonium salt.
[0103] Melting point range: 179-183°C (decomposes while melting)
[0104] amorphous solid
[0105] X-ray powder diffraction (diffraction angle (2θ°), () indicates relative intensity)
[0106] 6.02(25), 17.4(46), 17.4(53), 17.6(72), 17.9(62), 18.7(22), 18.8(23), 18.9(21), 20...
Embodiment 3
[0113] Dissolve 3 g (4.9 mmol) of oxidized glutathione in 7 g of water to prepare 10 g of an aqueous solution containing oxidized glutathione, and further add 0.33 g (4.9 mmol: molar ratio to glutathione) of 25% ammonia solution =1). The temperature of this aqueous solution (pH=3.5) was heated to 40° C., and 30 ml of isopropanol (volume ratio to the aqueous solution=3) was added thereto over 1 hour, and a solid was deposited. After maintaining the temperature at 40° C. for 30 minutes, it was cooled to room temperature, and the solid was recovered by filtration. The solid recovered by filtration was dried under reduced pressure to obtain 2.9 g (content: 96% by mass) of oxidized glutathione monoammonium salt.
[0114] The results of melting point, X-ray diffraction, and elemental analysis are the same as those of the oxidized glutathione monoammonium salt described in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com