Antipyrine derivative probe material for naked eye detections and identifications of Cr3+ and Cu2+ as well as preparation method thereof
A technology for aminoantipyrine and derivatives, which is applied in the measurement of color/spectral properties, organic chemistry, etc., can solve the problems of high cost, poor reagent selectivity, complicated equipment and other problems, and achieves high yield and simple and convenient post-processing. , the effect of a simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the synthesis of compound (3)
[0035] (1) Weigh 4g (24mmol) carbazole, add 20mL of anhydrous DMF, stir at room temperature until completely dissolved, then add 1g NaH in batches, seal in oil, continue stirring at room temperature for 20min, then add 4mL (28mmol) bromhexine dropwise with a constant pressure funnel Alkanes in DMF (10mL) solution, after dropping, reflux in 80°C water bath for 3 hours to stop the reaction, pour it into water after cooling, adjust the pH value to neutral with hydrochloric acid with a volume ratio of 1:1, let it stand still, precipitate the crude product, pump Filter and recrystallize from ethanol to obtain 4.59 g of white needle-like crystals, with a yield of about 76.1%. FT-IR (KBr, cm -1 ):3049(=C-H),2856-2953(-CH 2 ,-CH 3 ), 1459 (-CH 3 ), 1323 (-CH 2 -),1621-1594(structure of carbozle). 1 H-NMR (CDCl 3 ,400Hz):δ H 8.09(d, J=7.60Hz, 2H), 7.38~7.48(m, 4H), 7.21(d, J=8.00Hz, 2H), 4.28(t, 2H), 1.86(m, 2H), 1.33(m ,6H)...
Embodiment 2
[0038] Embodiment 2: The ultraviolet-visible absorption spectrometry of compound (3)
[0039] Accurately weigh the test sample compound (3) (4.7mg), dissolve it and transfer it to a 10mL volumetric flask, use acetonitrile solvent to make up the volume, and prepare a concentration of 1.0×10 -3 mol / L stock solution. Accurately pipette the stock solution for the next step of dilution, and prepare to a concentration of 1.0×10 -5 mol / L test solution. The ultraviolet-visible spectrum and fluorescence spectrum were measured respectively.
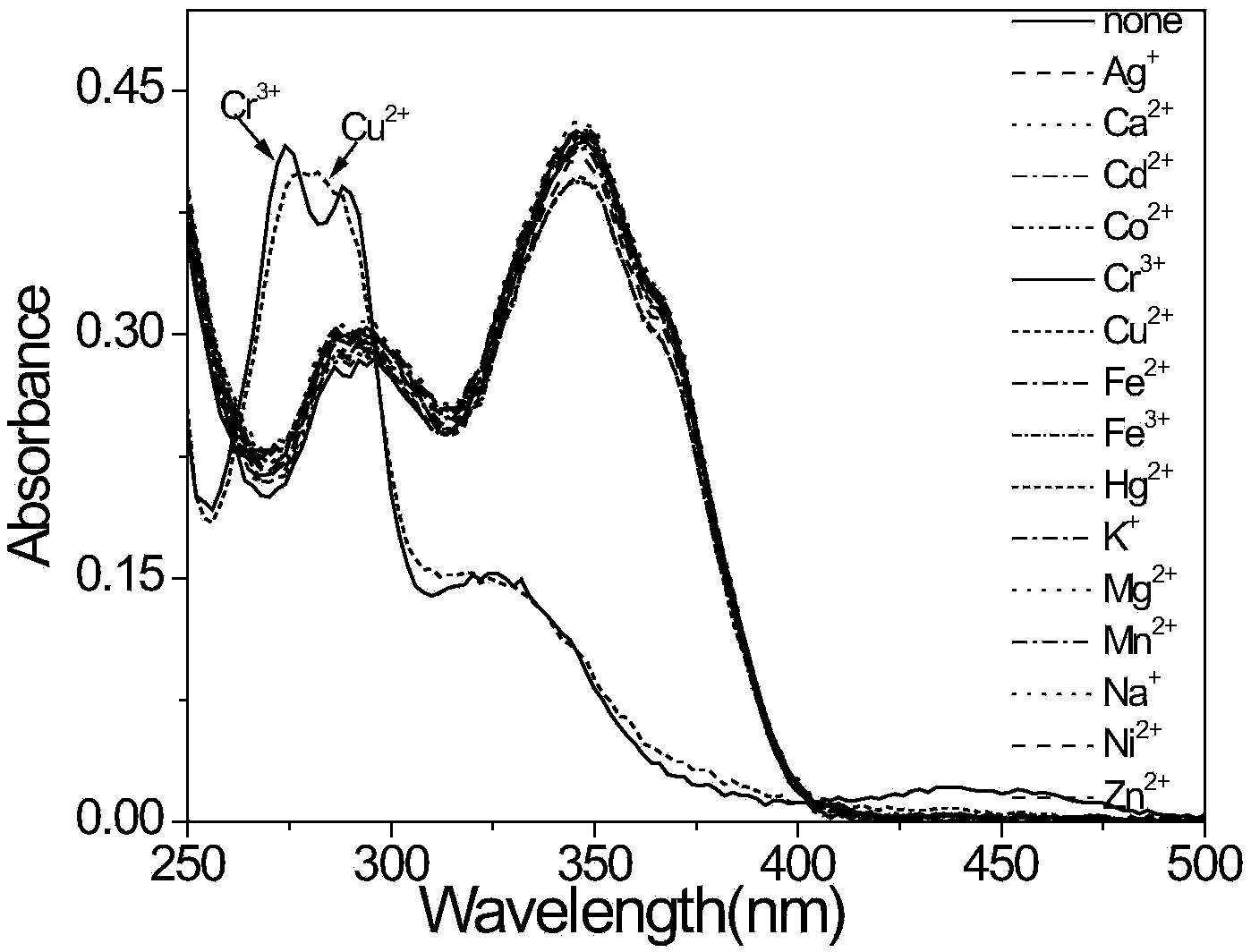
[0040] Take 3mL concentration as 1.0×10 -5 mol / L of the sample to be tested in a quartz cuvette (the thickness of the quartz cuvette is 1cm), and then add 30 μL of the concentration of 1.0×10 -3 mol / L stock solution of various metal ions, shake well and place for a certain period of time, and measure its influence on the probe absorption spectrum and fluorescence spectrum.
[0041] Various metal cations in the acetonitrile solution of compound...
Embodiment 3
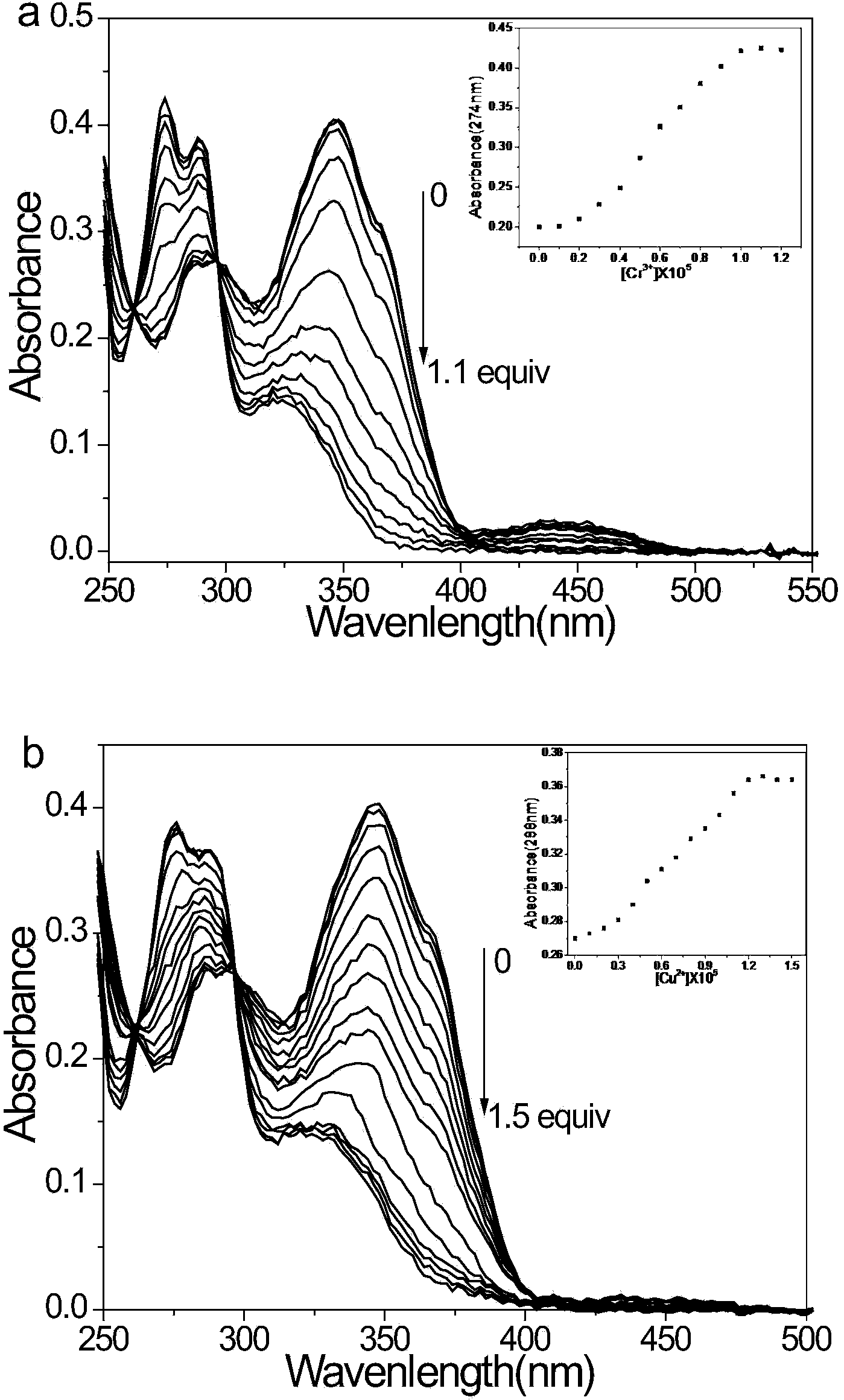
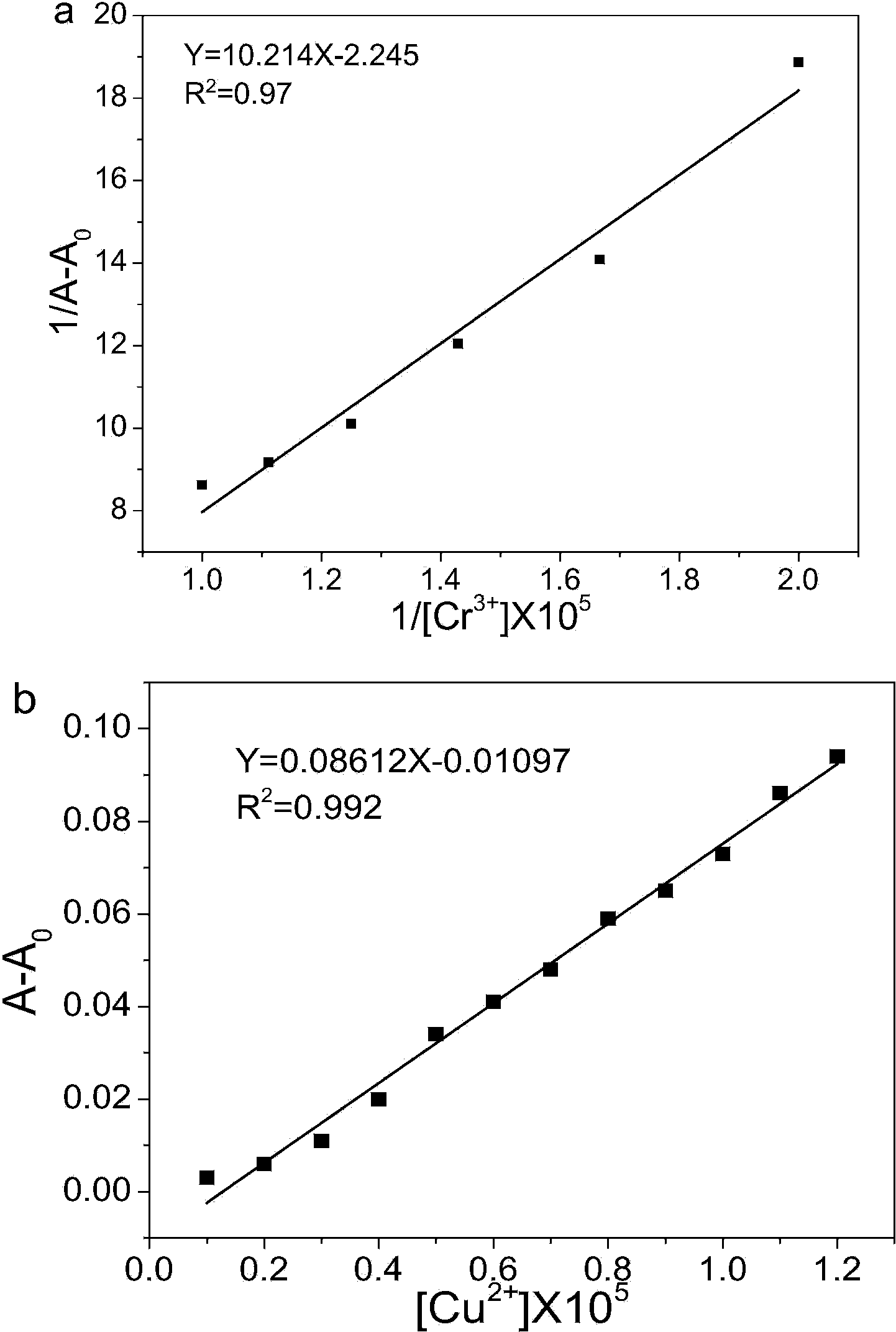
[0042] Embodiment 3: The determination of the ultraviolet-visible absorption spectrum titration experiment and detection limit of compound (3)
[0043] Cr 3+ and Cu 2+ The ultraviolet absorption spectroscopic titration experiment of the ion-pair probe compound (3) (as attached figure 2 shown), with Cr 3+ With the addition of ions, the acetonitrile solution of compound (3) gradually changed from colorless to yellow, and the absorbance of the shoulder peaks at 286nm and 293nm gradually increased, and the absorption peak gradually blue-shifted, and after the blue shift, it was still a shoulder peak (274nm and 287nm ), while the absorbance at 346nm gradually weakens, and the absorption peak gradually blue-shifts until the blue shifts to 322nm; with Cu 2+ With the addition of ions, the acetonitrile solution of compound (3) gradually changed from colorless to light yellow, and the absorbance of the shoulder peaks of compound (3) at 286nm and 293nm gradually increased, and the ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com