Preparation method of human D-CIK (dendritic cell activated and cytokine induced killer) cell with high toxicity and high value-adding capacity
A toxic and cellular technology, applied in the field of cellular immunology, can solve the problems of heterogeneous cell population proliferation ability and cytotoxic activity, etc., to achieve strong tumor cell killing activity, high cell expansion efficiency, and easy large-scale production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] In Example 1, the obtained PBMCs were isolated and cultured with D-CIK cells. Include the following steps:
[0039] 1. Collect 30-50 ml of peripheral venous blood from the patient, and obtain mononuclear cells by density gradient centrifugation of Ficoll-diatrizoate glucosamine. The specific steps are: 1500 rpm, centrifuge for 10 minutes, absorb the upper plasma layer, inactivate at 56°C for 30 minutes and centrifuge for later use, dilute the precipitated blood cells with normal saline, and divide human lymphocyte separation medium and diluted blood at a ratio of 1:2 Add the proportion into a centrifuge tube, centrifuge at 2000 rpm for 20 minutes, carefully absorb the buffy coat, wash twice with normal saline at 1600 rpm and 1300 rpm, and centrifuge for 7 minutes to obtain PBMCs .
[0040] 2. The above PBMCs were further separated to obtain mononuclear cells and suspension cells by the adherence method. The specific steps are: resuspend the above isolated PBMCs in PA...
Embodiment 2
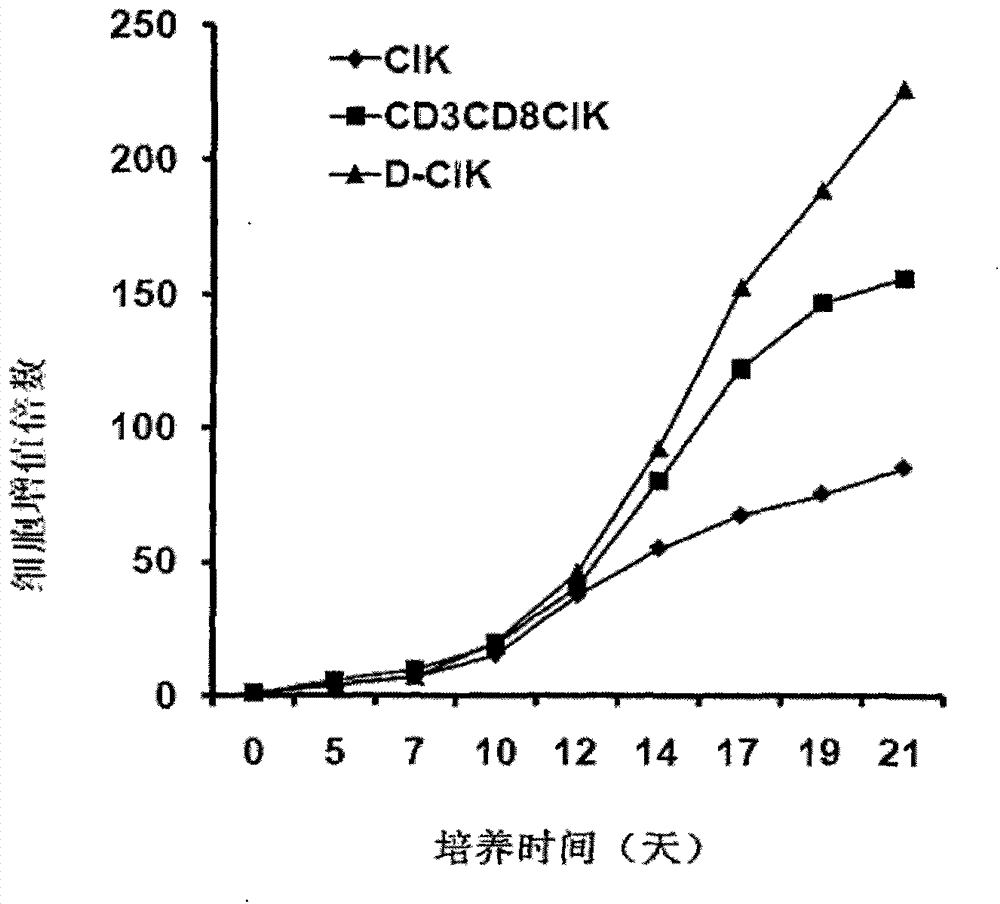
[0045] Example 2 is to detect the morphology and proliferation ability of D-CIK cells. Include the following steps:
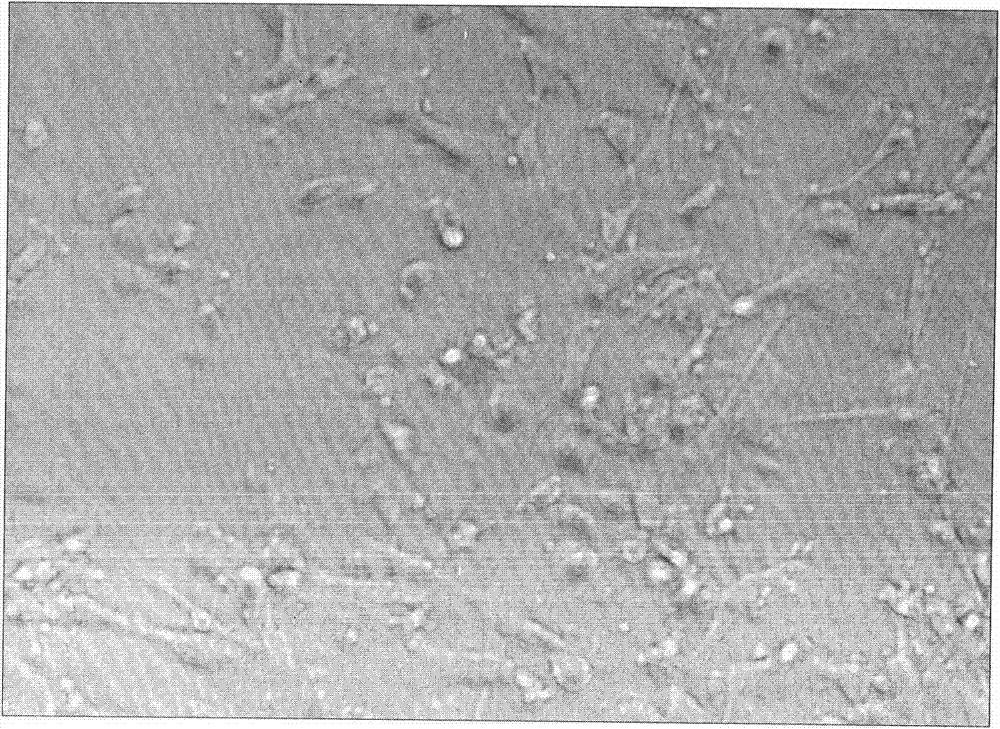
[0046] 1. DC and CD3+CD8+CIK were co-cultured for 24 hours, and the cells could be seen sinking to the bottom of the culture flask, with a large number of colonies of different sizes and irregular mononuclear cells. The cells cultured for 12 to 14 days were stained with Reggie's staining, and it was found that most of the cells were enlarged in size, oval or irregular in shape, most of the nuclei were oval or round, the nuclear staining was loose, and the nuclear membrane was irregular and protruding , a small nucleolus can be seen in each cell, which is dark blue; the cytoplasm is abundant, stained gray-blue, irregular in shape, with pseudopodia, light staining near the nucleus, and irregular in shape. Take 100 μl of cells cultured for 12-14 days, add 100 μl of 0.4% trypan blue staining solution, live cells are not stained, dead cells are stained blue, and co...
Embodiment 3
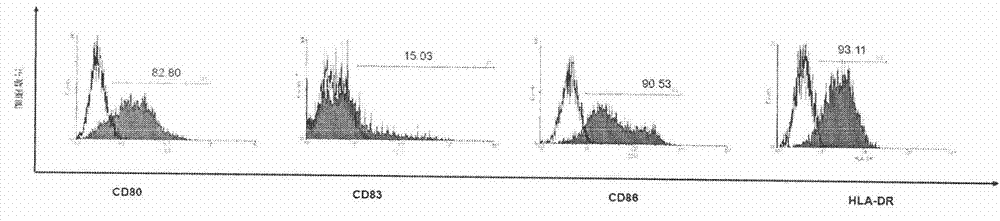
[0052] Example 3 is to detect the immunophenotype of D-CIK cells. Proceed as follows:
[0053] Take the cells on day 14, wash them twice with PBS containing 5% newborn bovine serum and 0.1% sodium azide, adjust the cell density to 1×106 / ml, add 50 μl of cell suspension to each detection tube, and add fluorescently labeled antibodies (anti-CD3-FITC, CD4-PE, CD8-PECY5.5, CD56-APC) staining, incubate at 4°C in the dark for 30 minutes, wash with the above PBS twice, add PBS solution containing 1% paraformaldehyde to fix, The flow cytometer was used for detection, and the data files were analyzed by WinMDI software. The results are shown in Figure 4 ,Table 2.
[0054] Table 2: Effects of different culture methods on the phenotype of PBMCs derived from peripheral blood (n=8)
[0055]
[0056]The results showed that on the 14th day of culture, there was no statistically significant difference among the CD3+ and CD3-CD56+ cells among the three groups of cells; the CD3+CD8+ and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com