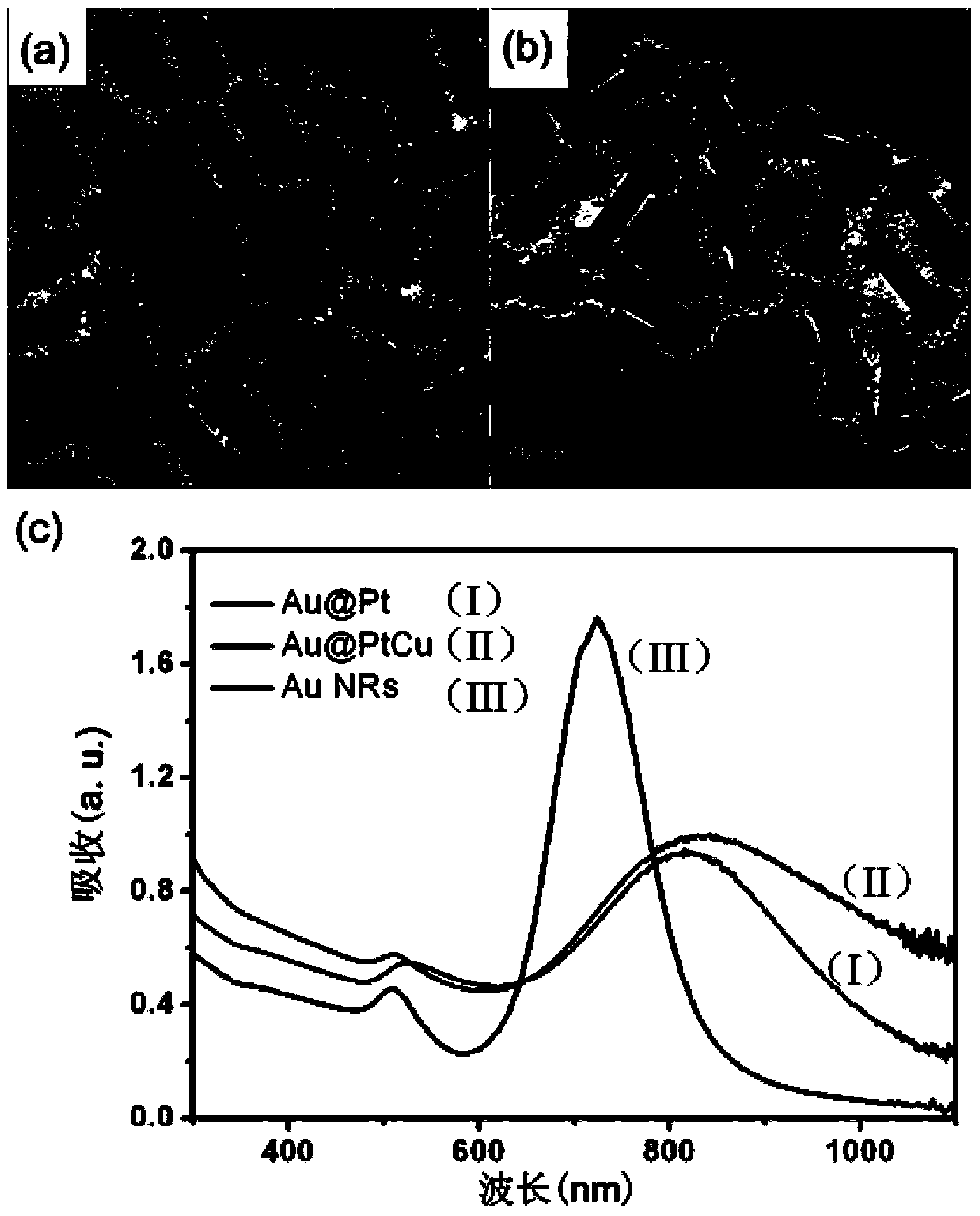
Platinum-based alloy structured nanorod simulation enzyme solution and application thereof in ELISA (Enzyme-Linked Immunosorbent Assay)
A platinum-based alloy, gold nanorod technology, applied in material inspection products, measuring devices, instruments, etc., can solve the problems of lack of in-depth research and reporting, and achieve the effect of simple and easy operation, easy preparation and high repeatability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Preparation and purification of nanorods with gold core / (copper) platinum alloy nano-island shell structure
[0050] The nanorods of the gold core / (copper) platinum alloy nano-island shell structure described in the present invention can be obtained by methods well known to those skilled in the art. In the present invention, we use gold nanorods as seeds to realize the preparation of nanostructures by reducing Pt with a reducing agent or co-deposition of Pt and Cu.
[0051] Preparation of gold core / platinum nano-island shell structure nanorods (AuPt): Take 1 mL of purified gold nanorod solution, add 75 μL of 2 mM K 2 PtCl 4 Solution, 22.5 μL of 0.1M AA solution, mixed evenly, placed in a 30°C water bath for half an hour, then added 0.5mL of 0.1M CTAB solution, centrifuged at 12000rpm for 5 minutes once, and the precipitate was dispersed in deionized water for later use .
[0052] Preparation of nanorods (AuPtCu) with gold core / copper-platinum alloy nano-is...
Embodiment 2
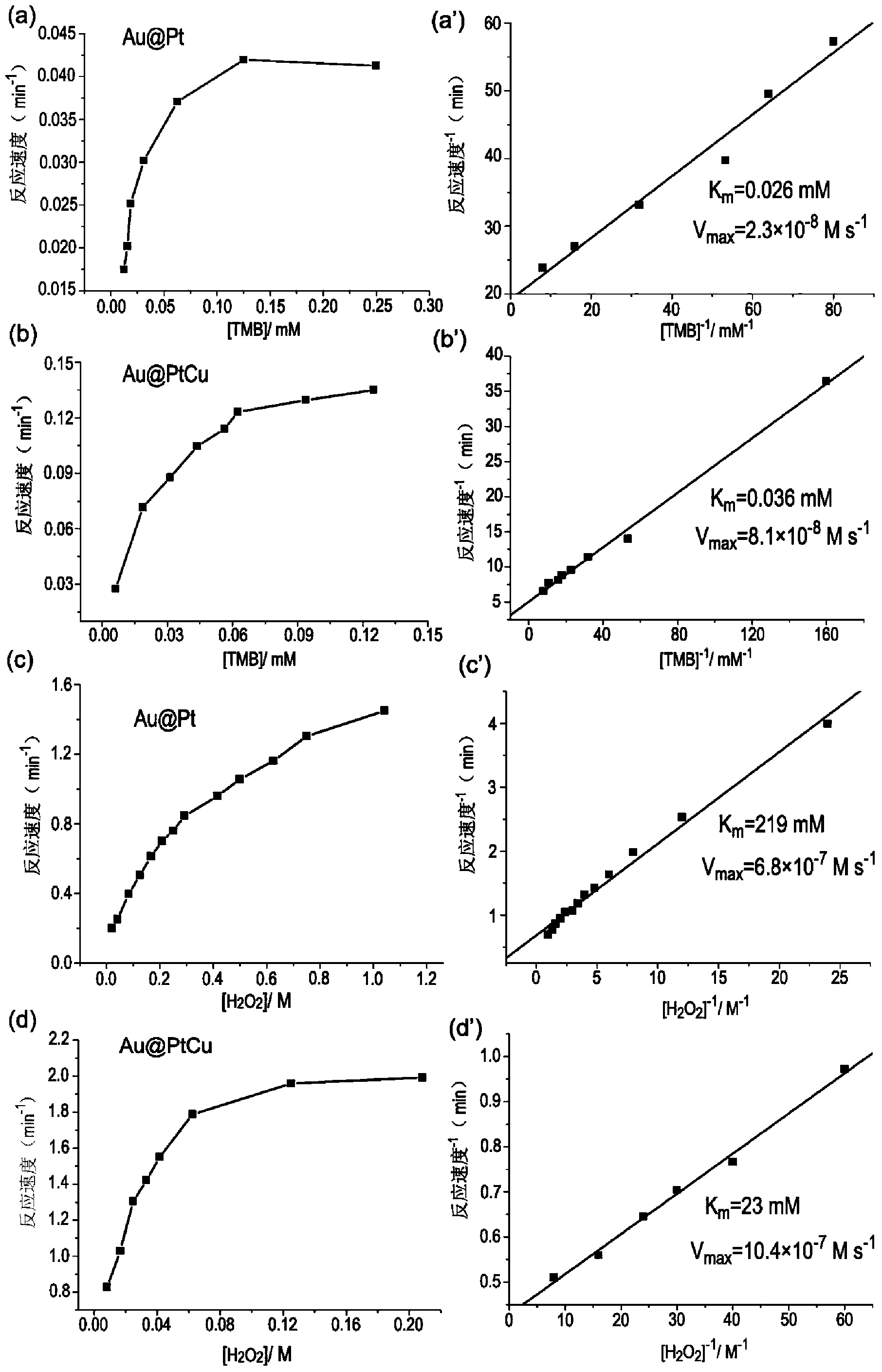
[0054] Embodiment 2: Enzyme kinetic parameter analysis
[0055] The reaction kinetics was investigated by monitoring the change of the absorbance of the TMB oxidation product. Using Varian Cary50 kinetic mode, every 0.1min interval measurement. The test conditions are: the concentration of AuPt or AuPtCu nanorods is 15pM, 2.4mL of 0.1M pH4.5 phosphate buffer solution, and the reaction temperature is set at 30°C. When using TMB as a substrate, H 2 o 2 The concentration is fixed at 2mM; when H 2 o 2 As the substrate, the concentration of TMB was fixed at 0.13mM. The apparent kinetic parameters are from the Lineweaver-Burk equation: 1 / V=(K m / V max )(1 / [C])+1 / V max get. where V is the reaction velocity, V max is the maximum reaction velocity, [C] is the substrate concentration, K m is the Michaelis constant.
[0056] The kinetic parameters obtained are shown in Table 1 and figure 2 shown.
[0057] Table 1 AuPt and AuPtCu nanorods as peroxidase-like apparent kinetic...
Embodiment 3
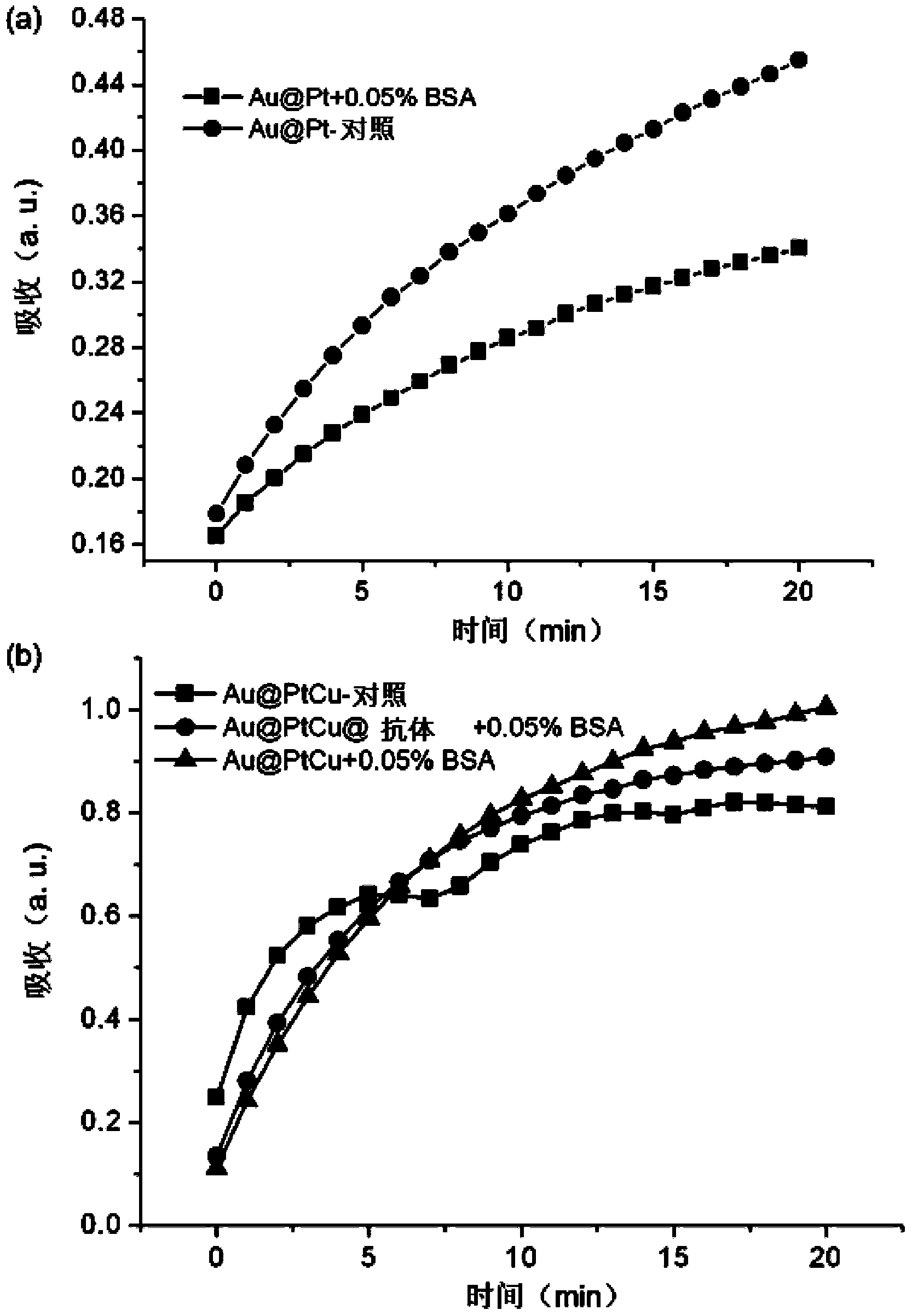
[0065] Example 3: Goat anti-human IgG antibody modification on the surface of nanorods with gold core / (copper) platinum alloy nano-island shell structure
[0066] Take 1 mL of the original CTAB-coated gold core / (copper) platinum alloy shell nanostructure solution that was purified once, add 50 μL of 20 mg / mL PSS solution, mix well, place it at room temperature for more than 3 hours, and then centrifuge at 12000 rpm for 5 minutes Once, the precipitate was dispersed in 200 μL deionized water for later use, and the concentration of the purified alloy nanorods was about 2 nM.
[0067] Take 1 mL of purified PSS-coated 2 nM alloy nanorod solution and disperse it in 1 mL of Tris buffer solution (0.05 M, pH 8) containing 0.05% BSA. Add 5 μL of 1 mg / mL goat anti-human IgG solution, mix well and put it in a 37°C incubator. After incubation for 30 minutes, centrifuge at 8000 rpm for 5 minutes to remove free goat anti-human IgG molecules. Pour off the supernatant, and disperse the precip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com