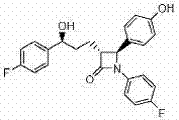
Preparation method of ezetimibe medicine composition
A technology of ezetimibe and composition, which is applied in the field of preparation of ezetimibe pharmaceutical composition, can solve the problems of easy aging, large preparation volume, and difficult acceptance by patients, and achieve the effect of large-scale production and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 One-step granulation of micronized ezetimibe
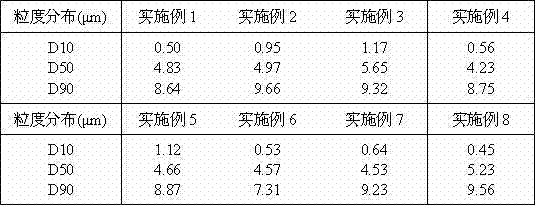
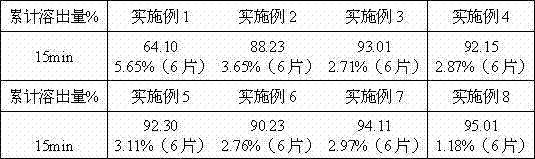
[0031] According to conventional methods, the ezetimibe API is first micronized, and the equipment used for the micronization treatment may be a jet mill, a ball mill or a fluid energy mill, or other equipment conventionally used in the art may be used. The particle size of ezetimibe should be controlled to be less than 10 μm by pre-crushing.
[0032] Formula (4000 tablets / batch)
[0033] No. Component Composition per tablet (mg) per batch (g) 1 Ezetimibe 10 40 2 lactose monohydrate 55 220 3 Microcrystalline Cellulose (Extra) 25 100 4 Croscarmellose sodium (additional) 3 12 5 Croscarmellose sodium (extra) 2 8 6 Povidone K30 3 12 7 Sodium dodecyl sulfate 1 4 8 Magnesium stearate 1 4
[0034] Preparation Process:
[0035] A. Dissolve the components of No. 6 and No. 7 in an appropriate amount of water to prepare adhesive solution I;...
Embodiment 2
[0041] Example 2 One-step granulation of micronized ezetimibe suspended in sodium lauryl sulfate solution
[0042] Prescription is with embodiment 1
[0043] Preparation Process:
[0044] A. Dissolve the component No. 7 in an appropriate amount of water to prepare binder solution I;
[0045] B. Suspend the micronized ezetimibe API (1) in the binder solution I, grind and mix evenly with a colloid mill to obtain the suspension solution II.
[0046] C. Add the components of serial numbers 2 and 4 into the fluidized bed and mix thoroughly to obtain mixture III;
[0047] D. Add the suspension solution Ⅱ to the mixture Ⅲ by spraying, granulate and dry to prepare granules Ⅳ; the fluidized bed parameters are set at 60°C for the inlet air temperature and 55M for the inlet air volume 2 / h, spray speed 4±2g / min.
[0048] E. Add the components of serial numbers 3, 5, 6, and 8 to Granule IV and mix evenly to prepare Granule V;
[0049] F. Compress granule V into tablets, the theoretic...
Embodiment 3
[0051] Example 3 One-step granulation of micronized ezetimibe suspended in sodium lauryl sulfate and HPMC E5 solution
[0052] Formula (4000 tablets / batch)
[0053] No. Component Composition per tablet (mg) per batch (g) 1 Ezetimibe 10 40 2 lactose monohydrate 55 220 3 Microcrystalline Cellulose (Extra) 25 100 4 Croscarmellose sodium (additional) 3 12 5 Croscarmellose sodium (extra) 2 8 6 HPMC E5 3 12 7 Sodium dodecyl sulfate 1 4 8 Magnesium stearate 1 4
[0054] Preparation Process:
[0055] A. Dissolve the components of No. 6 and No. 7 in an appropriate amount of water to prepare adhesive solution I;
[0056] B. Suspend the micronized ezetimibe API (1) in the binder solution I, grind and mix evenly with a colloid mill to obtain the suspension solution II.
[0057]C. Add the components of serial numbers 2 and 4 into the fluidized bed and mix thoroughly to obtain mixture III;
[0058] D. Add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com