Patents
Literature
359 results about "Dosing units" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
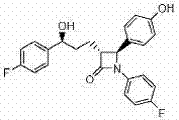
A unit dose is a method of preparing and packaging medications into single-use, pre-measured containers that provide exactly one dose.
Controlled-release drug delivery system
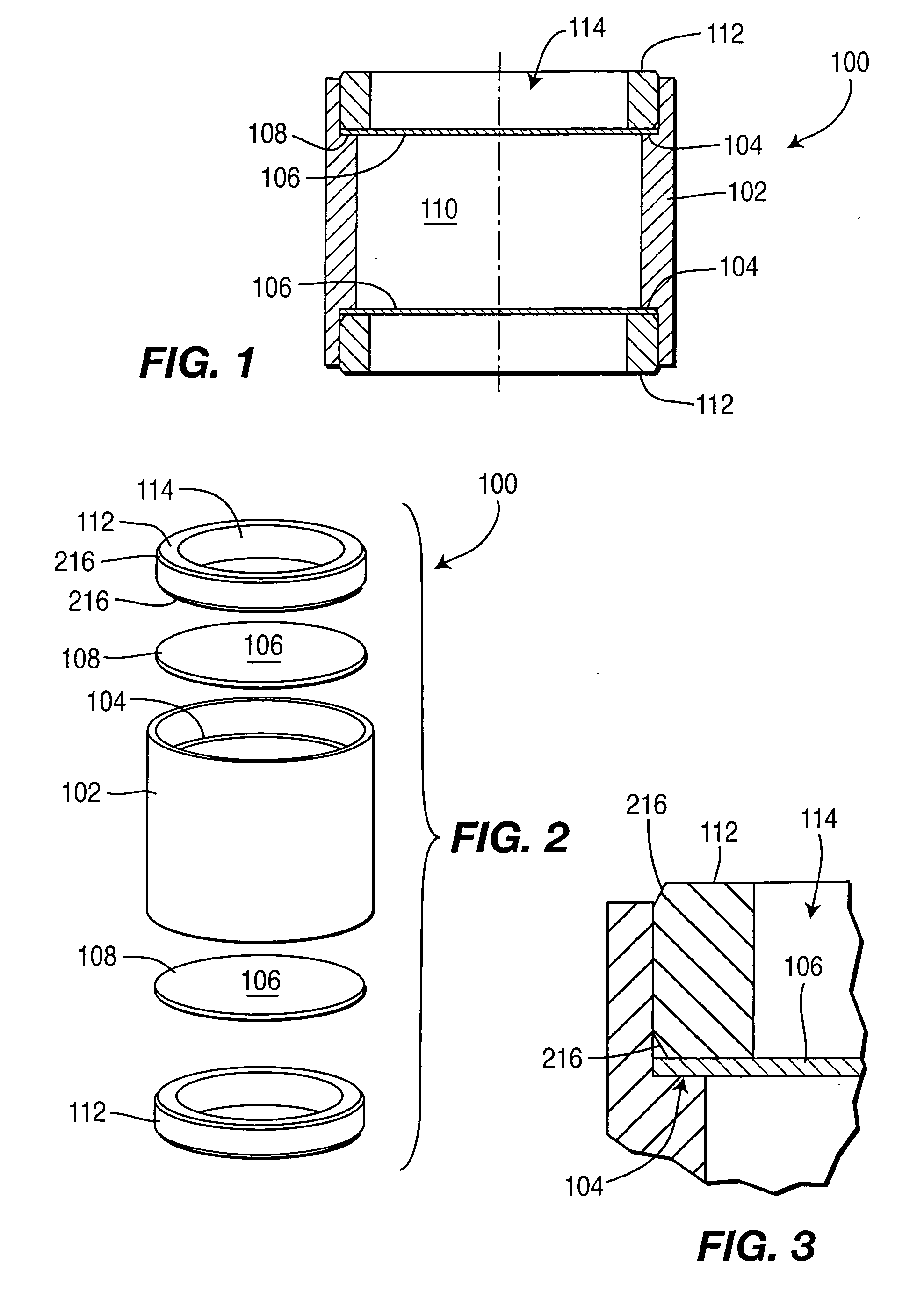
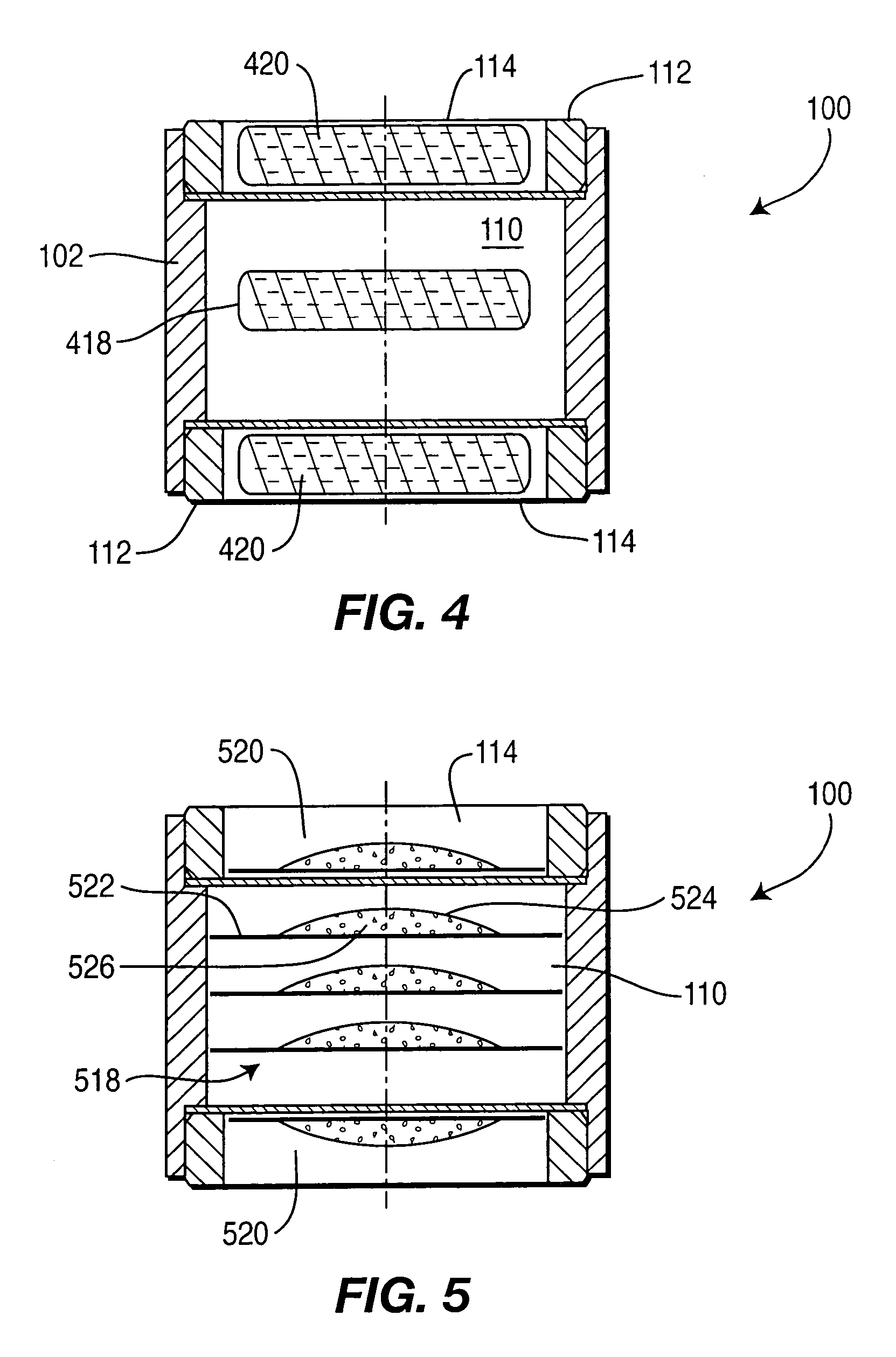
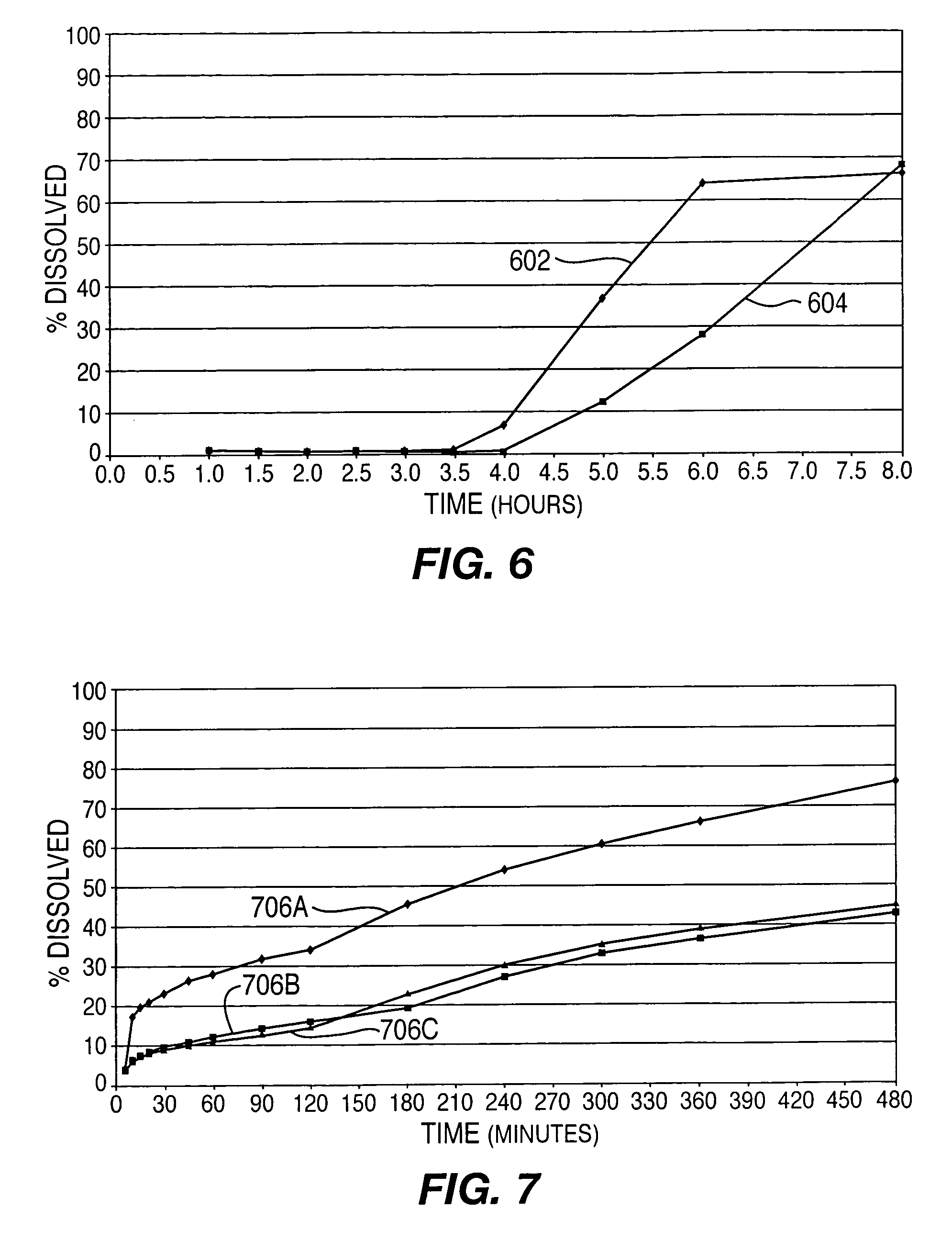
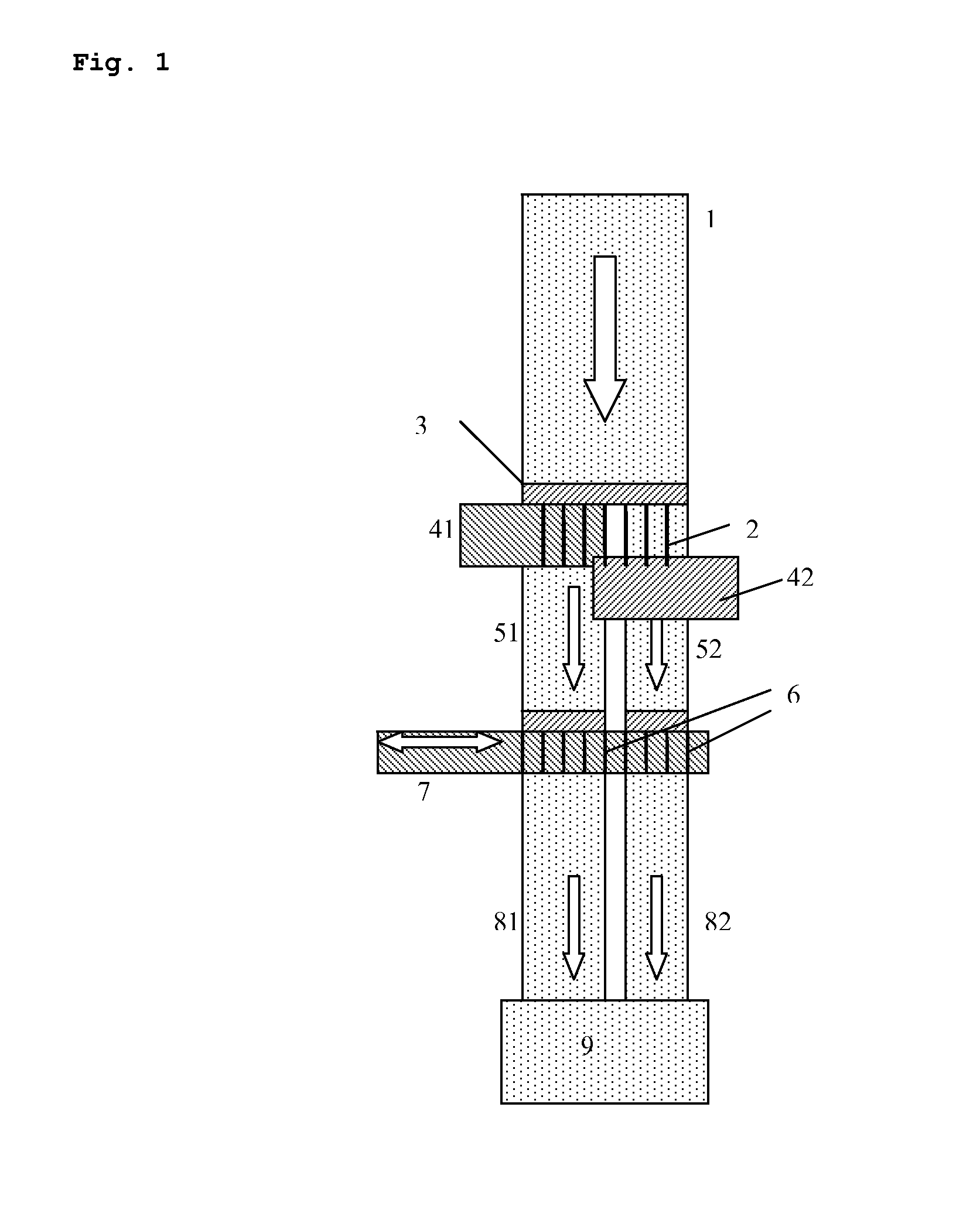
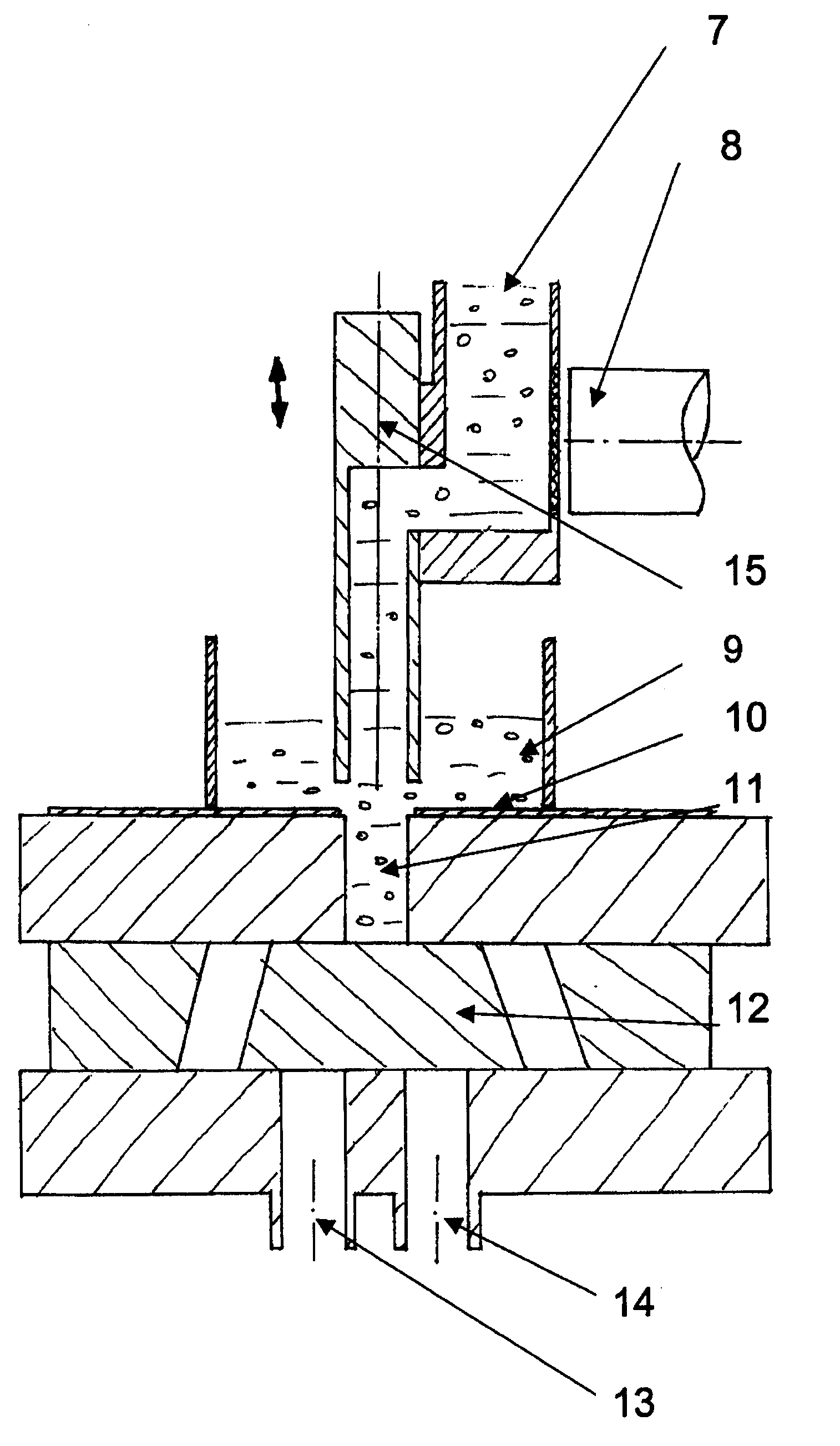
A controlled-release drug delivery system advantageously includes an open-ended, inflexible sleeve, at least two controlled-release layers and two open-center caps. Each controlled-release layer abuts a sealing surface that is located within and near each end of the sleeve. The caps seal each controlled-release layer against the abutting sealing surface. One or more dose units of drug are disposed in a region that is formed between the controlled-release layers. The controlled-release layers dissolve, at a predetermined rate, by the action of body fluids that are in contact with those layers through the center of the caps. Release of drug is delayed at least until the controlled-release layers dissolve. The dose unit itself, which is advantageously a core, can be tailored to provide an extended period of drug release. One or more dose units that provide an immediate release component can also be disposed near each end of the sleeve.
Owner:SARNOFF CORP
Pharmaceutical preparation containing a gestagen, and kit and method for treating endometriosis using the preparation
InactiveUS20080214512A1Significant positive effectEndometriosis can be reducedOrganic active ingredientsBiocideSide effectBone density
The pharmaceutical preparation for treating endometriosis contains at least 28, preferably 30, daily dose units, each of which contain dienogest, cyproterone acetate, or chlormadinone acetate at a daily dose that is at most twice that required to inhibit ovulation together with one or more pharmaceutical aids and / or carriers. The daily dose units are administered in a method of prophylaxis and / or therapy of endometriosis continuously during a time interval of at least 169 days or 25 weeks, preferably more than two years. The method effectively reduces endometriosis and associated pain, while undesirable side effects including bone density decrease are reduced or eliminated.
Owner:BAYER SCHERING PHARMA AG
Method and device for dosing and packaging polysilicon chunks and dosing and packaging unit
ActiveUS20120198793A1Low costAccurate doseWrapper twisting/gatheringSolid materialHard metalProcess engineering
Disclosed is a method for dosing and packaging polysilicon chunks, wherein a product flow of polysilicon chunks is transported via a feed channel, separated by at least one screen into coarse and fine chunks, weighed and dosed to a target weight by a dosing balance, discharged via a discharge channel and transported to a packaging unit where a first plastic bag is filled with the polysilicon chunks and sealed, the plastic bag containing polysilicon chunks being packaged with a further plastic bag which is formed by a shaper and subsequently welded, wherein the at least one screen and the dosing balance at least partially include a hard metal on their surfaces and the shaper for forming the plastic bag includes a wear-resistant coating. Also disclosed are a dosing unit, a packaging unit and a device for dosing and packaging polysilicon chunks, which contains a dosing unit and a packaging unit.
Owner:WACKER CHEM GMBH
Method and device for vaporization and inhalation of isolated substances
A dose unit comprising at least one isolated bioactive agent applied on a carrier material in thermal contact with an electrically heating element configured to vaporize a pre-determined amount of the agent for pulmonary delivery thereof is provided herein, as well as devices for effecting vaporization and pulmonary delivery of the isolated agent, and methods for preparing the dose unit, controllably releasing the agent therefrom, methods for pulmonary delivery thereof and methods of treatment of medical conditions treatable by pulmonary delivery of the isolated bioactive agent.
Owner:SYQE MEDICAL
Process for producing a water-soluble package containing a composition
ActiveUS7469519B2Minimized volumePacked tightlyCapsDecorative coversPolymer scienceFilling materials
A process for producing a substance with a water-soluble packaging. The process comprises the following steps: a) a water-soluble material is deformed so as to embody a vessel; b) the vessel is filled with a filling material selected among the group comprising detergents, cosmetics, pharmaceuticals, body care products, auxiliary agricultural agents, adhesives, surface-treating agents, construction materials, dyes or food items; c) a water-soluble film web is applied to the filled container; d) the filled container and film web are sealed; and e) the sealed and filled container is finished. The inventive methods are characterized in that a negative pressure is generated in the filled container in the course of the process. In order to generate the negative pressure, the air located between the filling material and the water-soluble film web applied in step c) escapes at least in part through openings in the water-soluble film web applied in step c). The inventive methods make it possible to produce compact dosing units that have a reduced volume while being provided with improved optical and haptic properties.
Owner:HENKEL KGAA
Apparatus and method for electro chemical deposition
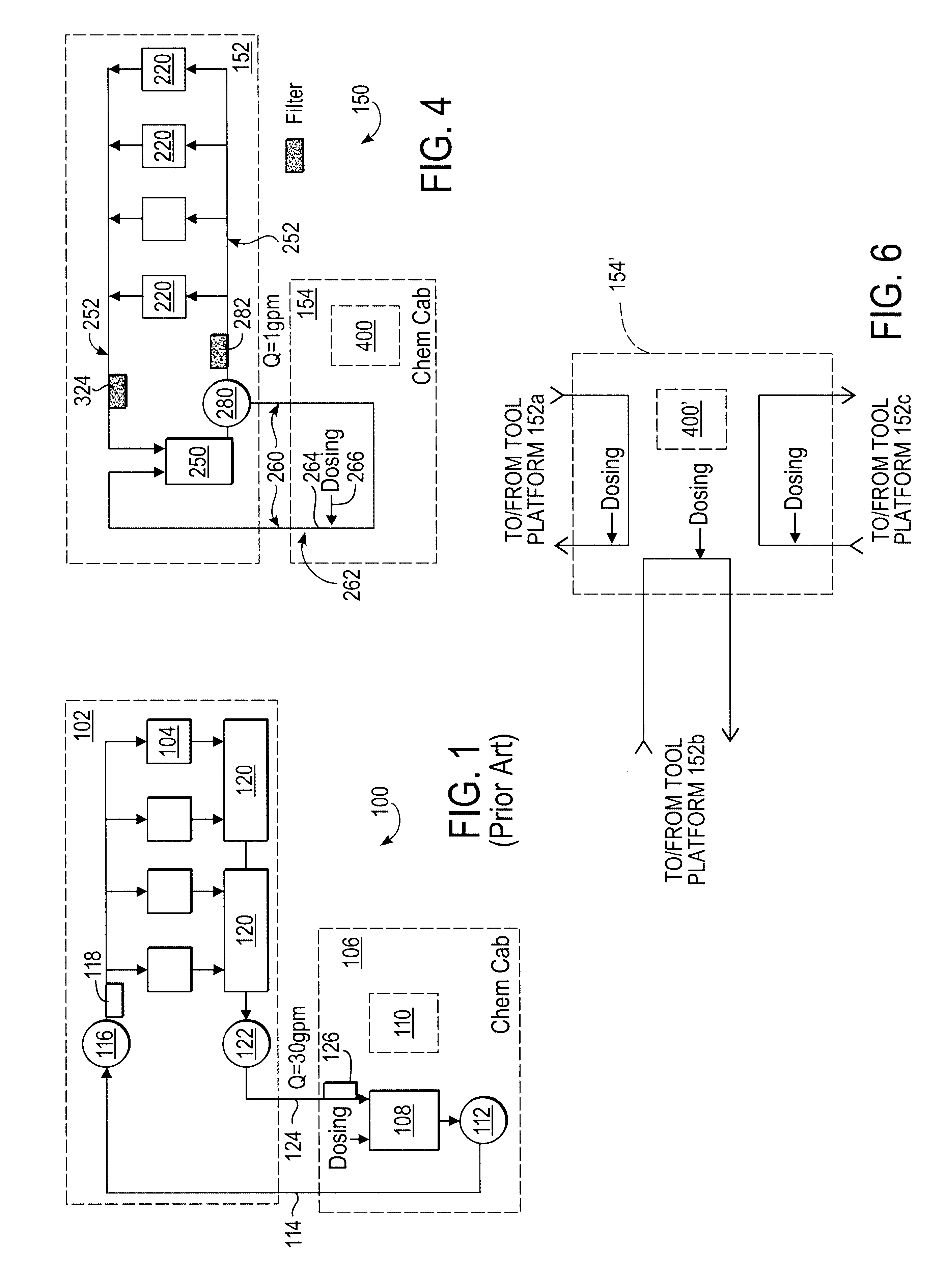
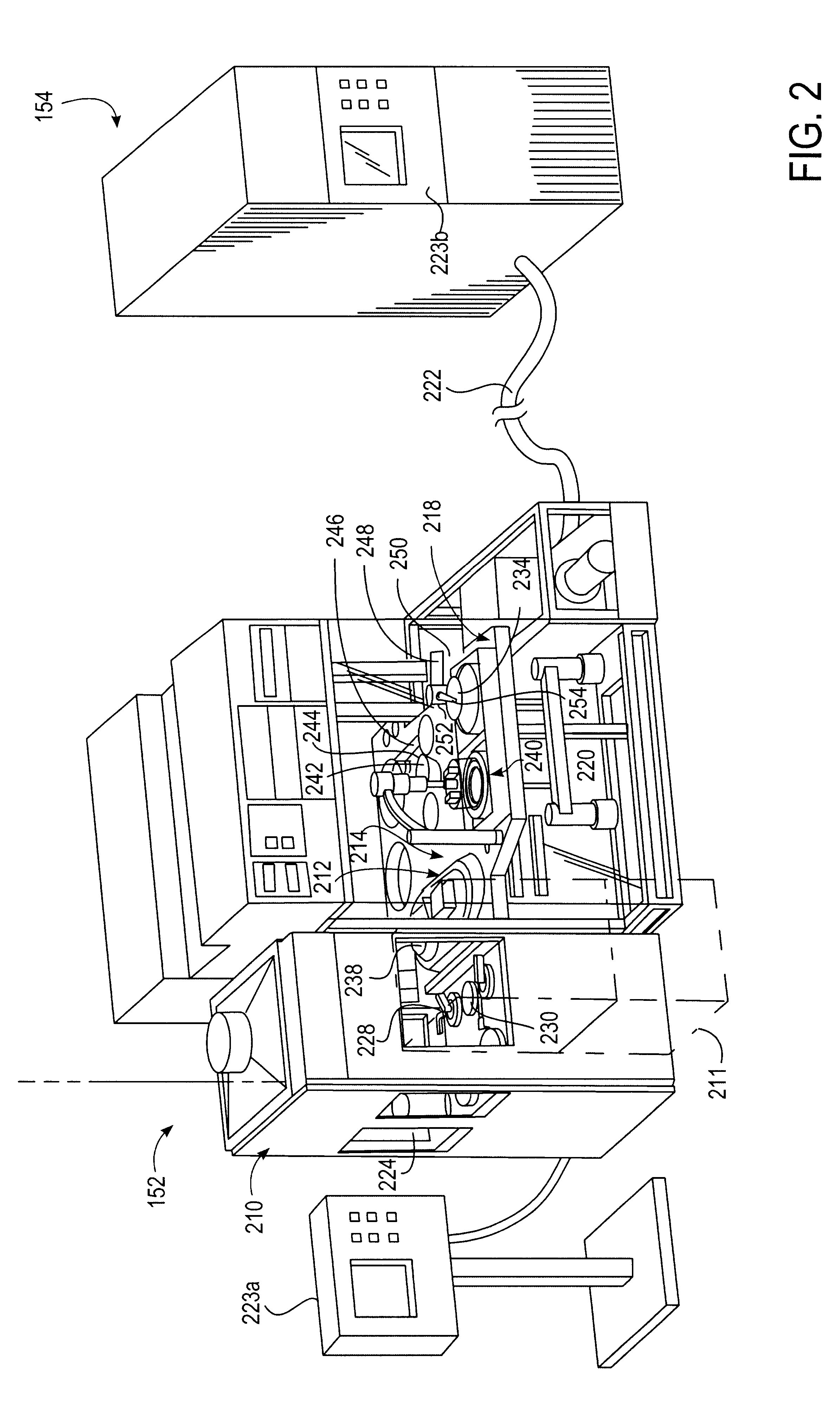
A system is provided in which a smaller flow of deposition solution is diverted from a larger flow of deposition solution flowing on an electro-chemical deposition tool platform. The smaller flow is diverted to a dosing unit which may be on a separate platform. The dosing unit in one embodiment comprises a pressurized flow line.
Owner:APPLIED MATERIALS INC
Method and device for vaporization and inhalation of isolated substances
Owner:SYQE MEDICAL LTD
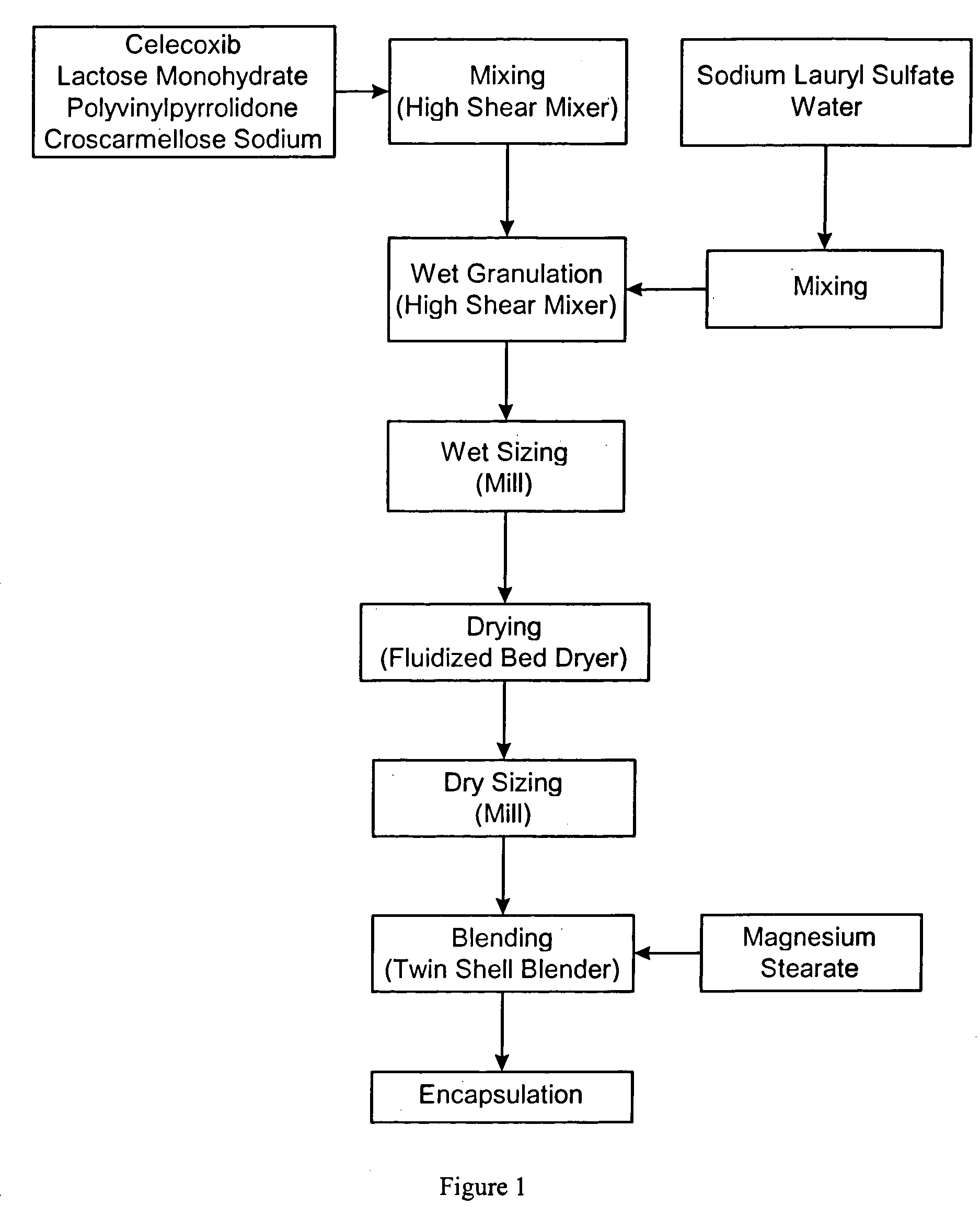
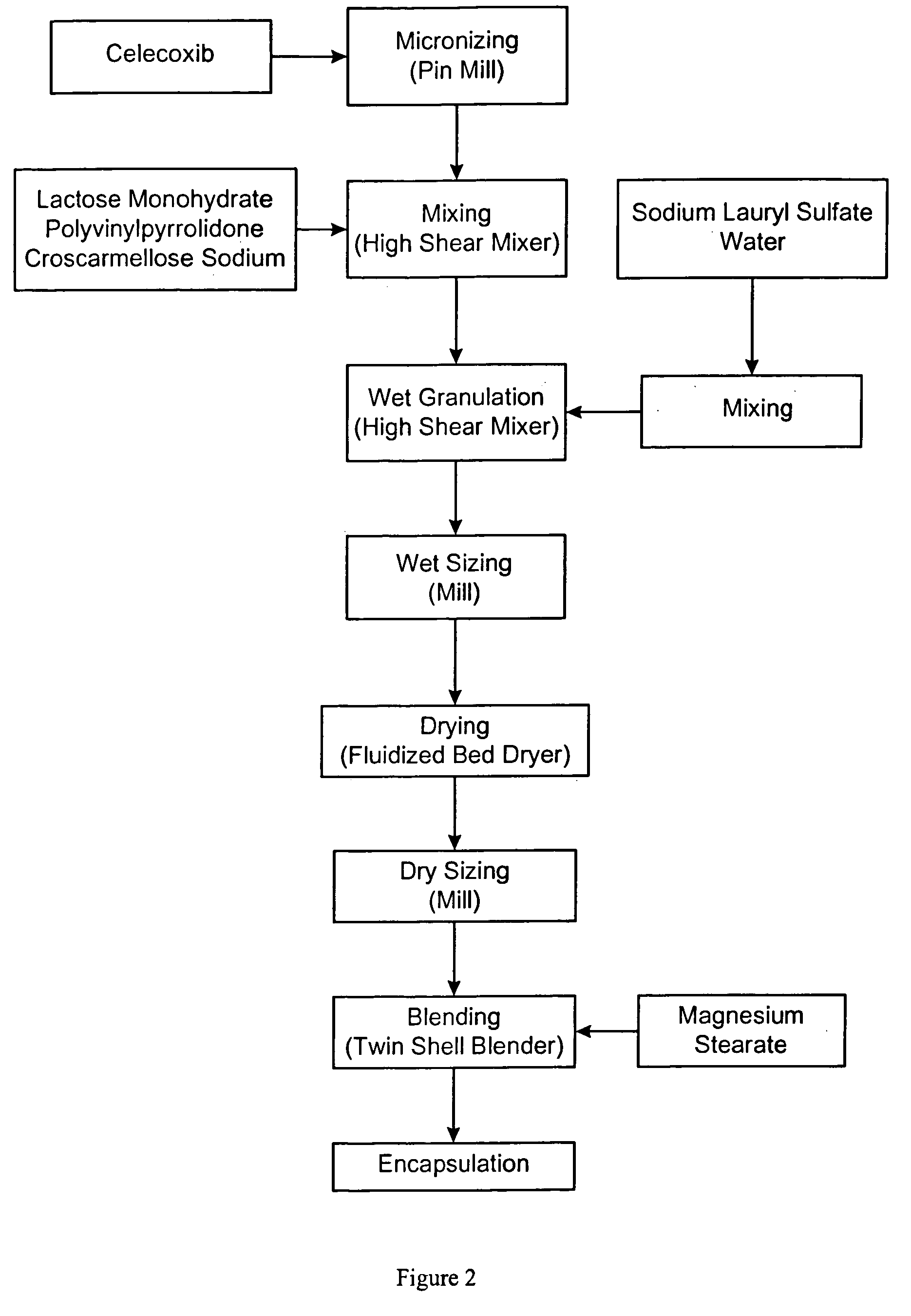
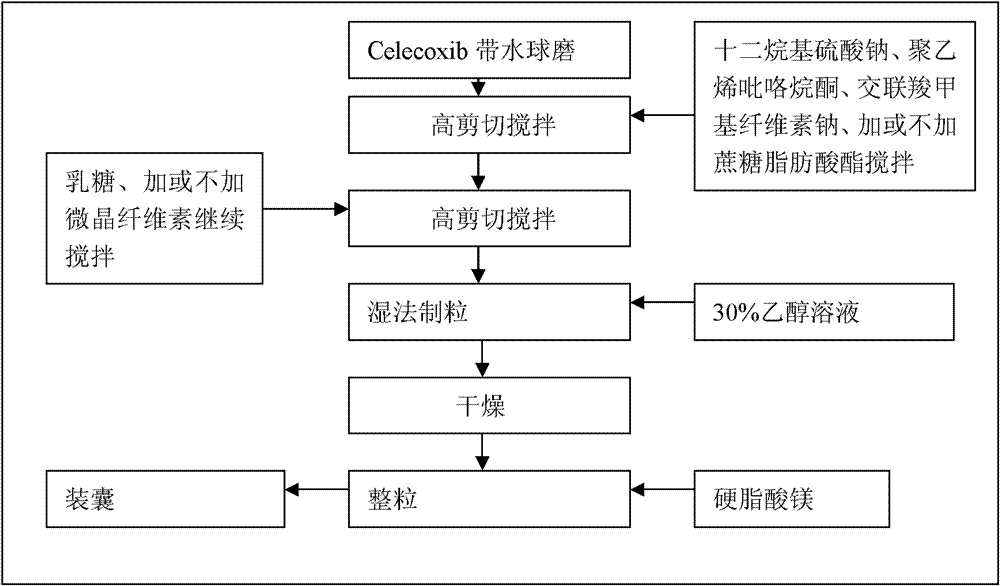
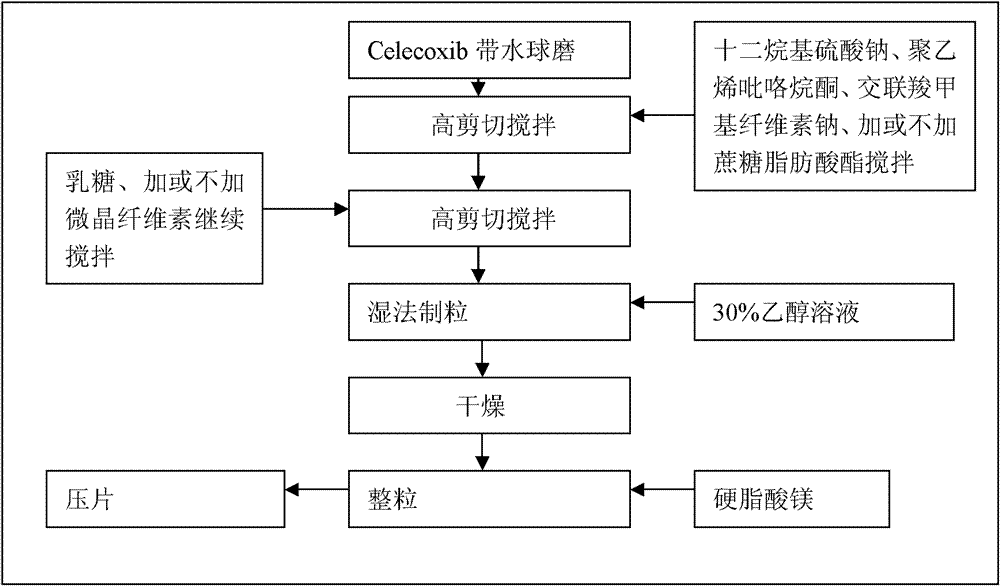
Celecoxib compositions
InactiveUS20050267189A1BioavailabilityLess-harmful side effectBiocideSenses disorderParticulatesCyclooxygenase
Pharmaceutical compositions are provided comprising one or more orally deliverable dose units, each comprising particulate celecoxib in an amount of about 10 mg to about 1000 mg in intimate mixture with one or more pharmaceutically acceptable excipients. The compositions are useful in treatment or prophylaxis of cyclooxygenase-2 mediated conditions and disorders.
Owner:GD SEARLE & CO
Preparation method of ezetimibe medicine composition
InactiveCN103655453AEasy to operateEasy to scale up productionOrganic active ingredientsMetabolism disorderMedicineEzetimibe
The invention relates to a preparation method of an ezetimibe medicine composition. The preparation method comprises the following steps: 1, suspending ezetimibe in a proper solvent to prepare an uniform ezetimibe micron-sized suspension; 2, adding the suspension into a diluent of the medicine composition in a spraying manner and drying to prepare particles and powder of the ezetimibe medicine composition; and 3, preparing the particles and the powder into the ezetimibe medicinal minimum dose unit. Firstly, the medicine is prepared into the micron-sized suspension, granulation is carried out in a one-step granulation manner, the medicine is added into the diluent and finally, the medicinal minimum dose unit is prepared. The preparation method has the benefits that the effect of the preparation method is superior to that of a method in which micronization is carried out after the slightly soluble medicament is separately micronized; and the operation is simple and the large scale production is easy.
Owner:CHINA RESOURCES SAIKE PHARMA
Method and System
InactiveUS20070169836A1Minimize doseRisk minimizationSolid materialCapsule deliveryDosing unitsPackaging machine
The present invention relates to a method for dosing a pharmaceutical product in a packaging machine comprising at least one volumetric dosing unit with a dosing chamber. The method comprises metering a volume of a first component, weighing the first component, introducing the first component into a package and metering a volume of a second component, introduceg the second component into the package and weighing of the package into which the components have being introduced.The invention further relates to a system for dosing a pharmaceutical product in a packaging machine comprising at least one volumetric dosing unit with a dosing chamber. The system comprises at least one volumetric dosing unit for metering a volume of the components. Further, means for introducing the said components into a package and means for weighing the components and the package are comprised. The means for weighing comprises a first weighing unit for weighing the package and the components, and a second weighing unit for weighing the first component after metering the first component by volume and before introducing the first component into the package.
Owner:ASTRAZENECA AB
Liquid delivery system with horizontally displaced dispensing point
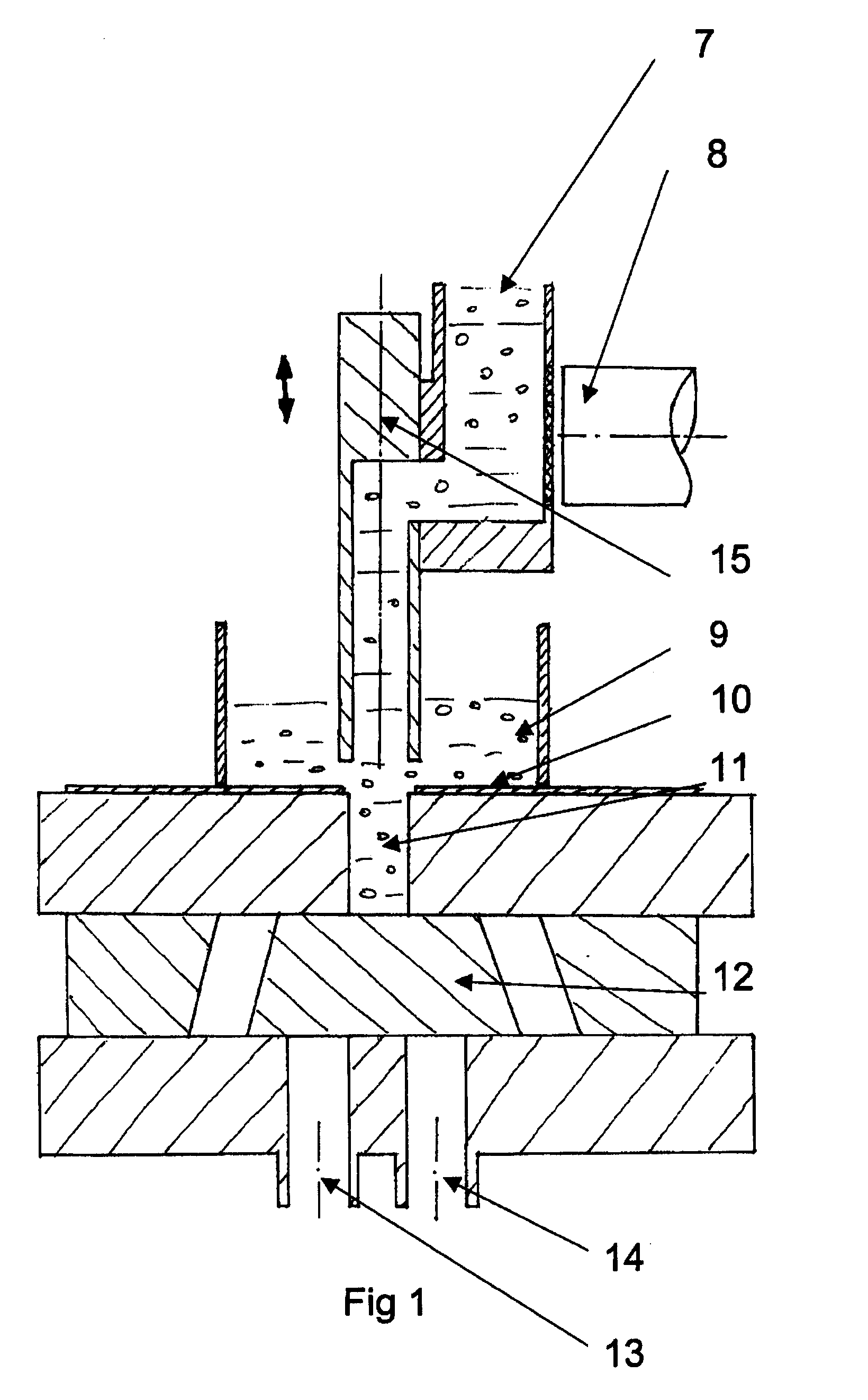
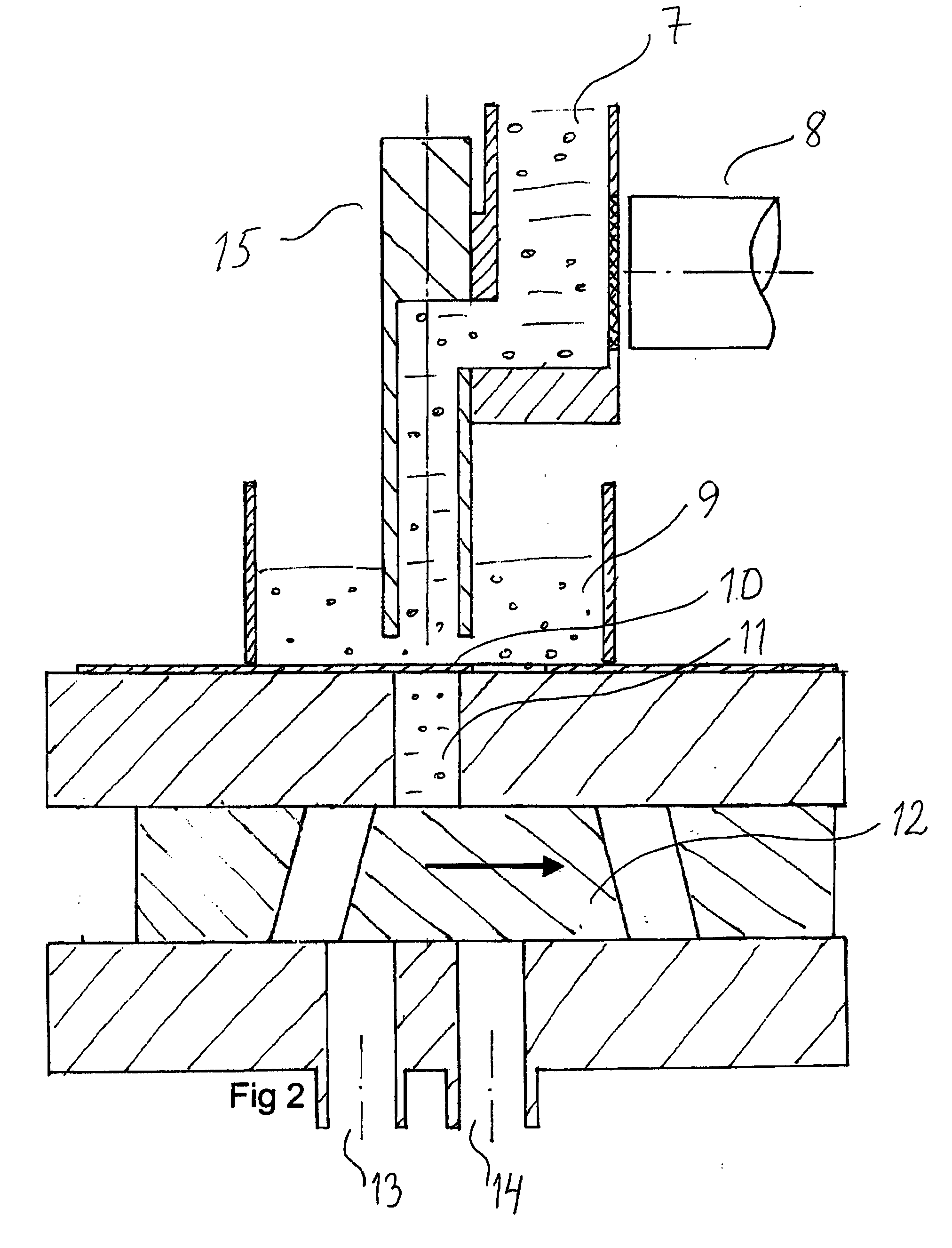
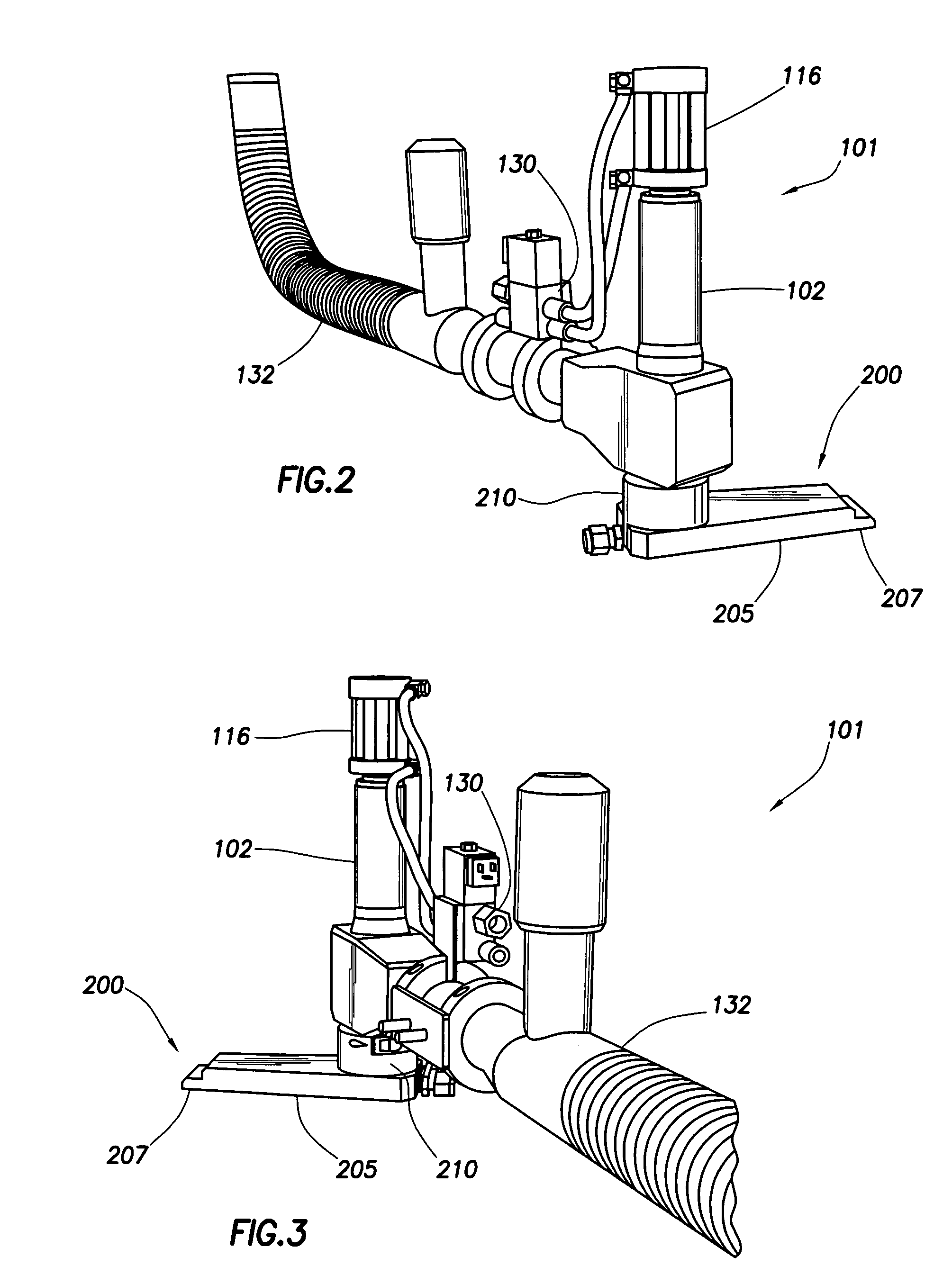
A method and apparatus is provided for the efficient and controllable delivery of cryogen liquid droplets into thin walled containers before they are sealed, the pressurization of the sealed container caused by the evaporation of the liquid cryogen causing the walls of the container to stiffen. Discharge of the droplets immediately upstream of the container sealing station is facilitated using a horizontal displacement assembly to transport metered droplets from a liquid dosing unit to the point of injection above the container. The horizontal displacement assembly may be provided with internal heaters to prevent freeze up, and a sensor to confirm droplet discharge. It may also be provided with a separate source of heated nitrogen gas, which can be used to back purge the dispensing unit should it become clogged, to melt any frozen liquid occlusions which may have formed in the cryogen supply line. In one embodiment, the solenoid used to actuate the piston regulating the opening and closing of the needle valve, which meters the dispensing of droplets, is mounted in thermal contact with said piston, this placement of the solenoid serving to cool the piston and thus prevent overheating in the case of rapid cycling.
Owner:CHART INC
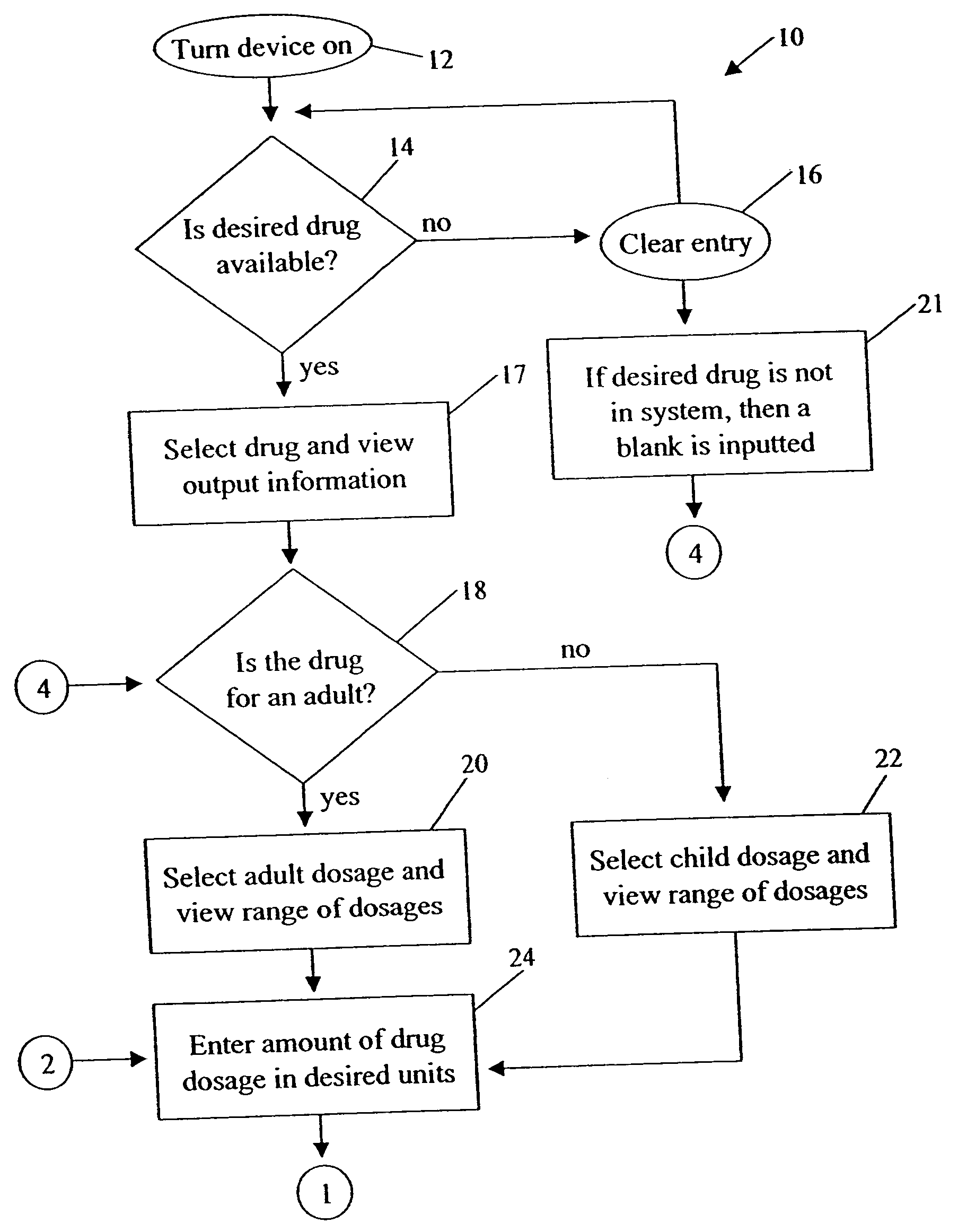
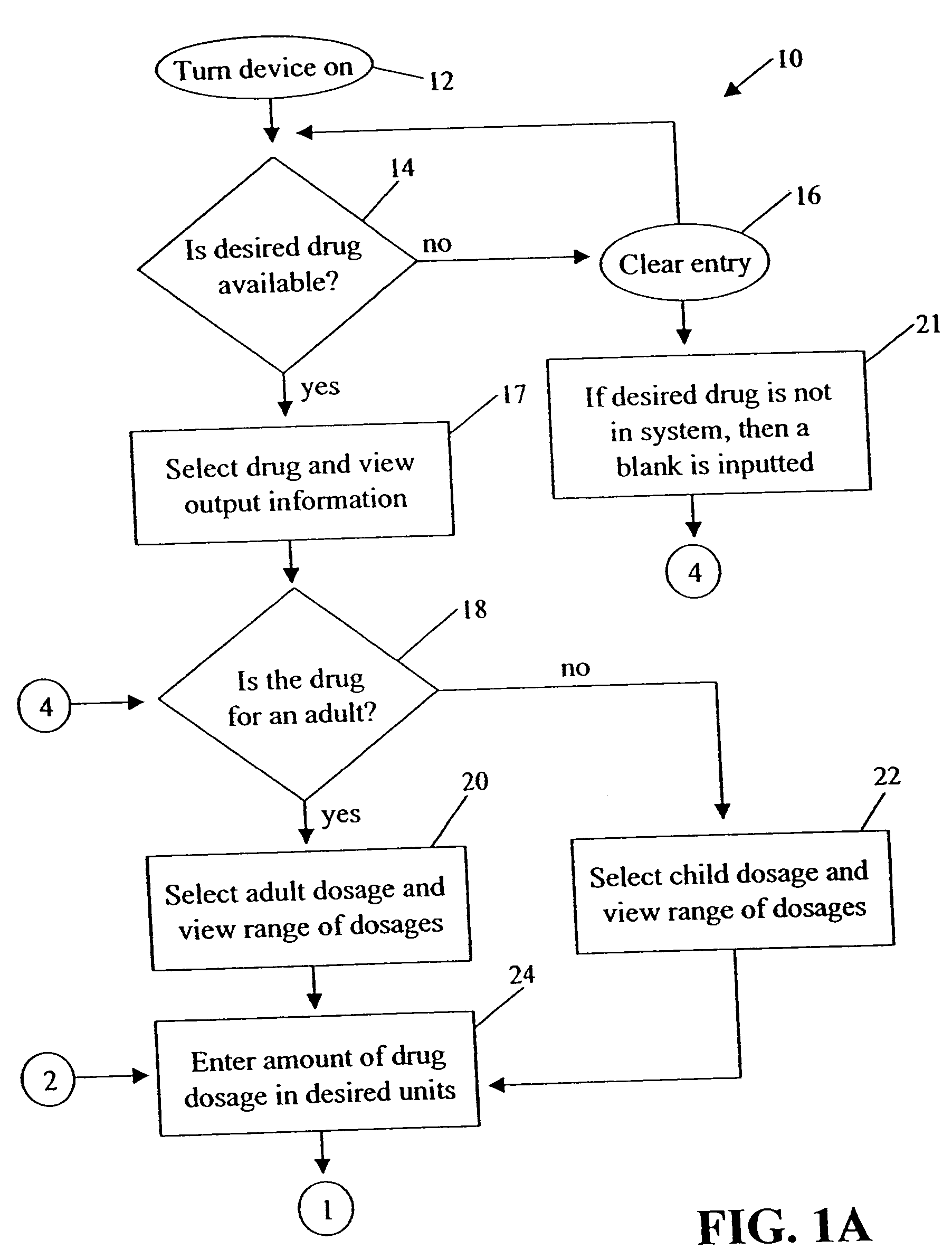
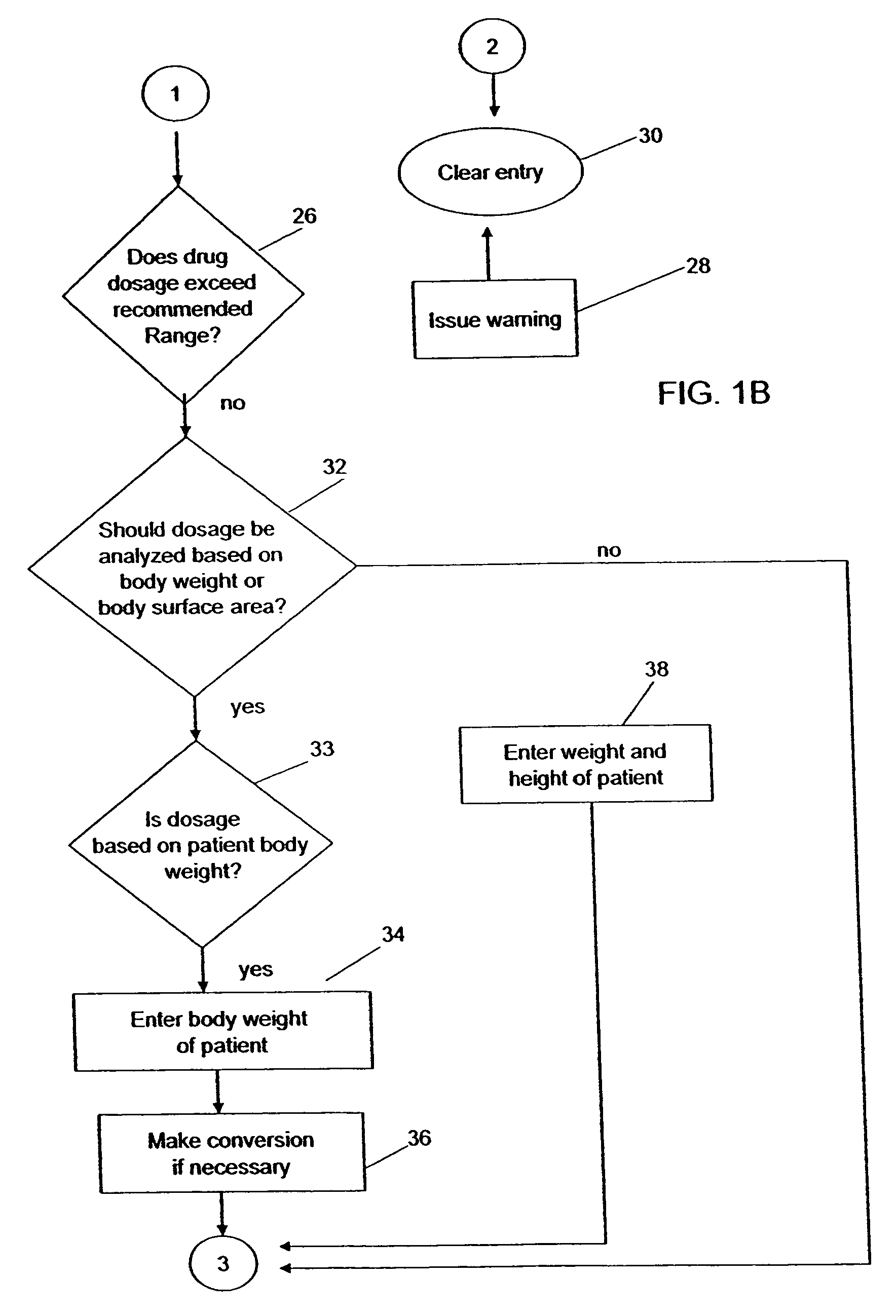
Medication dose calculator
InactiveUS20060129357A1Reduce needData processing applicationsDrug and medicationsMedication doseMedicine
A medication dose calculator and method for comparing an inputted, ordered medication dosage with a known medication dosage range in a database including an input device for inputting a desired drug name, indicating whether the drug is for a child or an adult, an amount of the drugs that are ordered, the body weight or body surface of the patient, and a computing mechanism for determining the dose of the drug to be delivered. The medication dose calculator provides warnings when the inputted amount of drug exceeds the dosage range limits or is incorrect. The medication dose calculator converts an inputted drug unit of measure into a desired unit of measure. In addition, various methods for receiving, recording, storing, transmitting, transferring, and editing information along with various processes utilizing the medication dose calculator are disclosed.
Owner:INFORMMED
Method and system for dosing a pharmaceutical sample in a packaging machine
InactiveUS7536843B2Securing of qualityImprove accuracyLiquid fillingSolid materialBiomedical engineeringDosing units
The present invention relates to a method and system for dosing a pharmaceutical product in a packaging machine having at least one volumetric dosing unit with a dosing chamber. The system check-weighs the volumetrically dosed product with appropriate speed and is integrated into a filling or packaging machine. In accordance with the invention, a volume of a first pharmaceutical component is metered and weighed before being introduced into a package. The procedure is repeated with a second pharmaceutical component. The package containing the components is then weighed. Advantageously, accuracy and precision of the amount of the weighed components can be monitored with the present invention to secure the quality of the pharmaceutical product.
Owner:ASTRAZENECA AB
Weeding procedure for a railway vehicle
The invention mainly provides a connection device for a weeder installed on a rail vehicle, which includes a weed identification camera, a PC connected to the weed identification camera for identifying weeds, and a meteorological unit. The units are all connected to the control PC. . The control PC is connected to the control panel, and the control panel is connected to the spraying unit through the electronic control unit and the electronic dosing unit. The spray unit includes a water tank, a pump, a flow meter, a control valve, a spray rack with a shaft and a nozzle, and a chemical tank, a chemical pump, and a chemical flow meter, all of which are connected together. The connection device also includes a species recognition camera connected to the species recognition PC, a landscape tracking laser camera connected to the landscape tracking PC, and a pre-spraying unit and a spraying unit. In addition, the present invention also mainly provides a method for weeding using the above-mentioned connecting device.
Owner:G & G NOVENYVEDELMI ES KERESKEDELMI
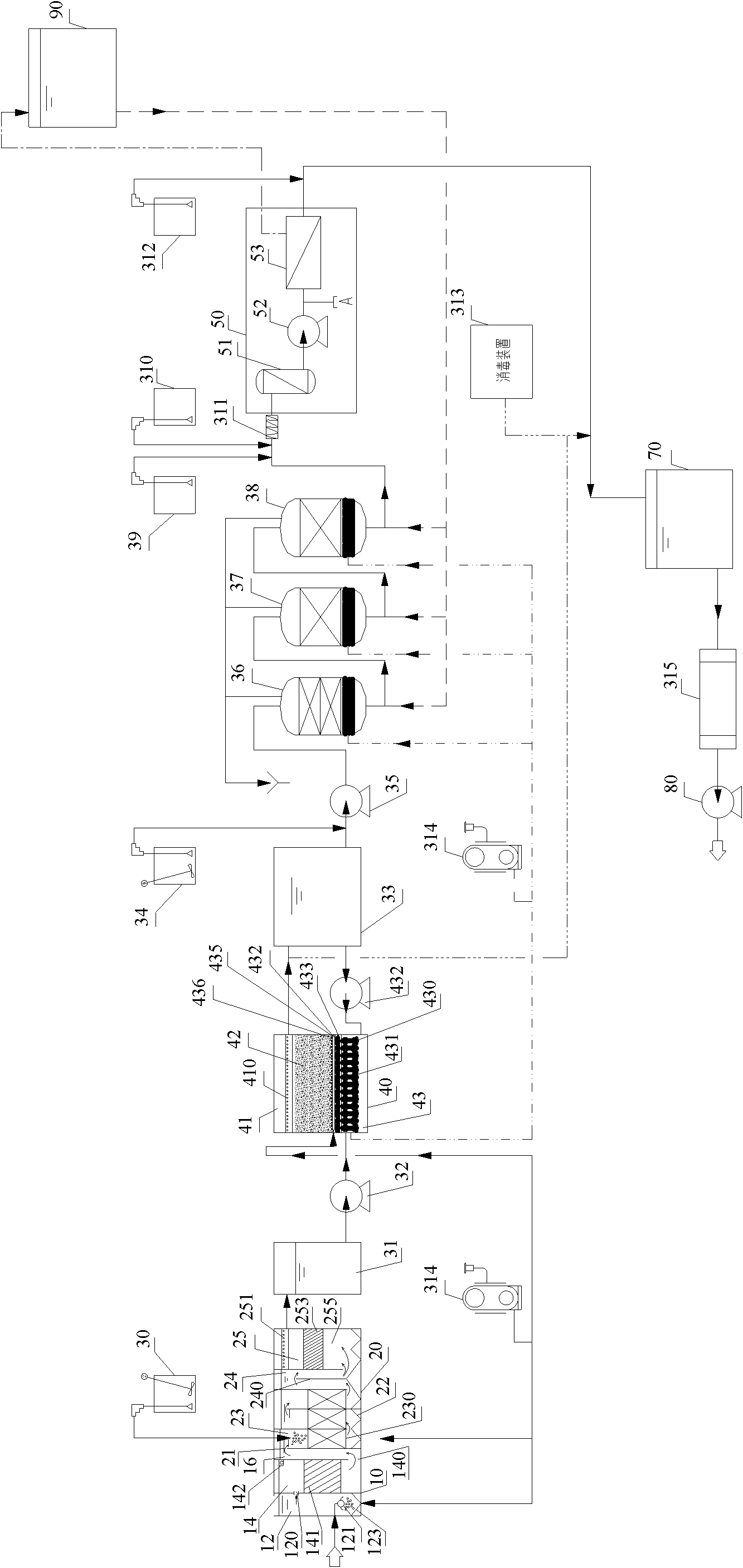
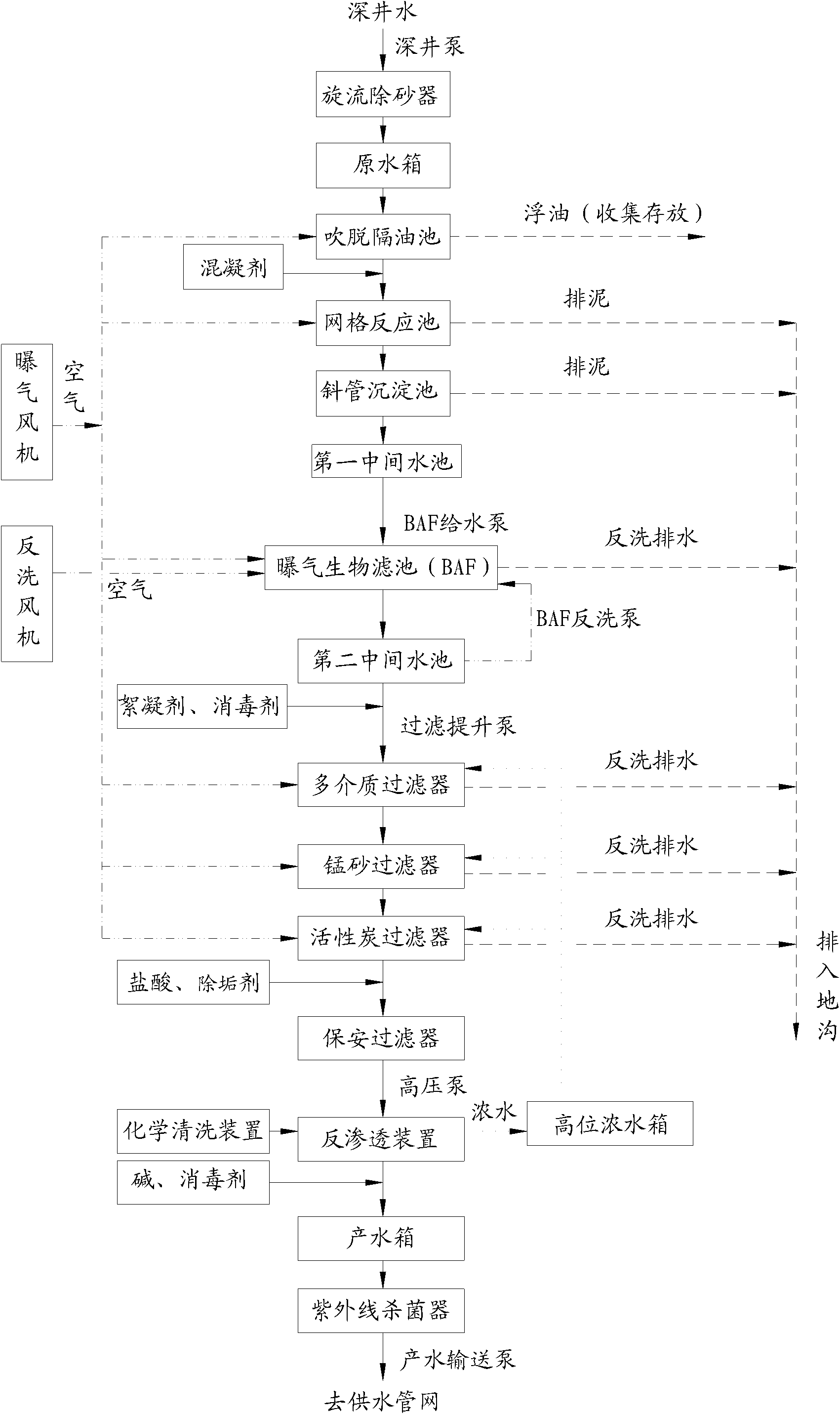
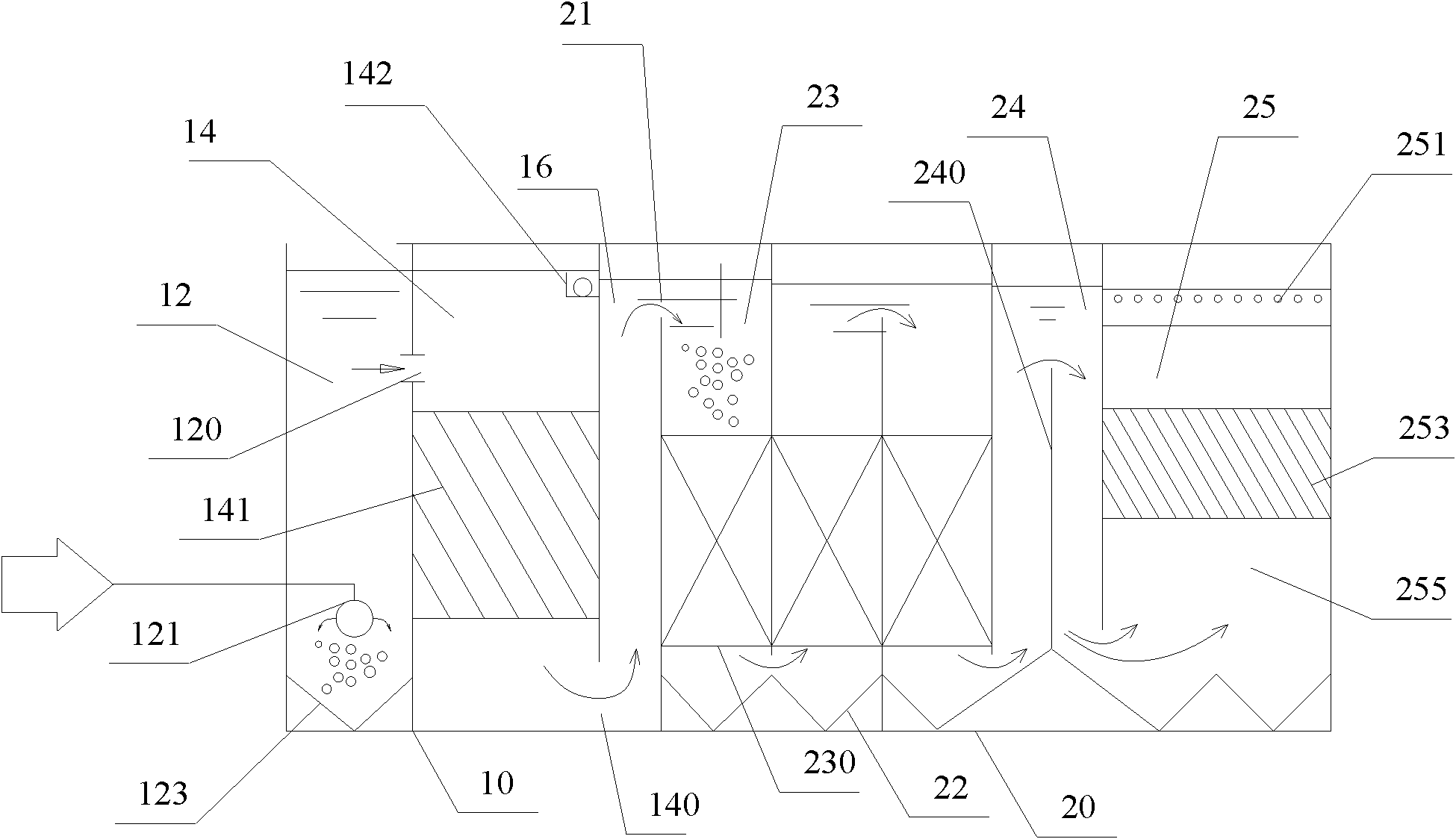
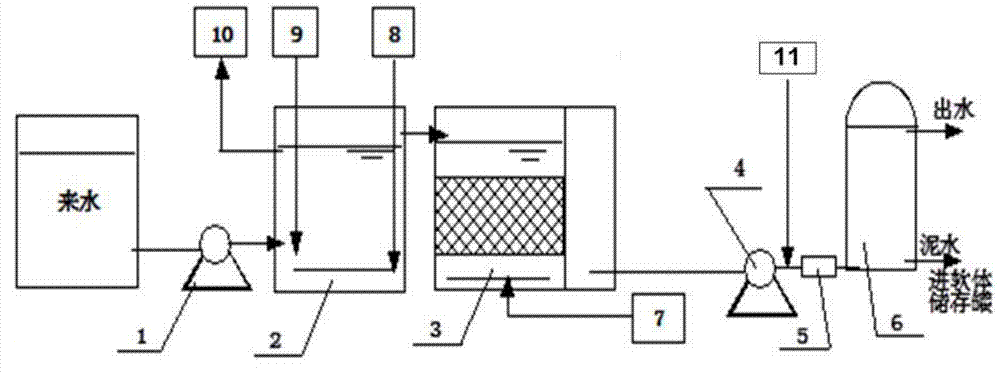
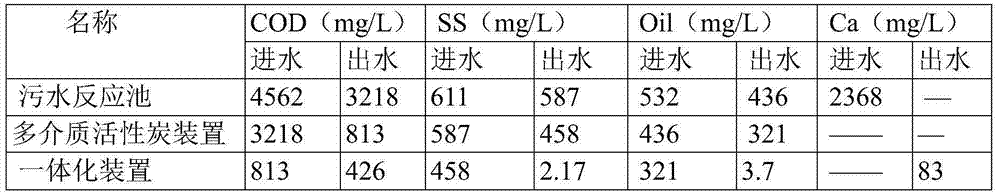
Purification system for micro-polluted raw water and purification method thereof
InactiveCN102557350AImprove recycling ratesStrong shock adaptabilityWater/sewage treatment by irradiationWater/sewage treatment bu osmosis/dialysisPurification methodsActivated carbon filtration
The invention relates to a purification system for micro-polluted raw water, comprising a stripping oil separation tank, a coagulating sedimentation tank, a coagulating chemical dosing device, a first middle water tank, a water feeding pump, a biological aerated filter, a second middle water tank, a coagulating chemical dosing unit, a filtering lift pump, a multi-media filter, a manganese sand filter, an active carbon filter, an acid dosing device, a scale inhibitor dosing device, a mixer, a reverse osmosis device, an alkali dosing device, a disinfection device and an ultraviolet sterilization device. The purification system for micro-polluted raw water disclosed by the invention has the following advantages that: main pollutants are especially removed at first, and then a reverse osmosis technology is used as a safety guarantee; the reverse osmosis pre-treatment is designed adequately, and the operation of the system is safe and stable, so that the occurrence frequency of pollution and blockage phenomena is greatly decreased, chemical cleaning period is greatly prolonged, and the recycling rate of water is up to 75%; and the purification system is suitable for the occasions of treating the drinking water of municipal administration, villages, industrial enterprises and mine fields or for the occasions of reusing the reclaimed water which needs advanced treatment. The invention further refers to a purification method of the purification system for micro-polluted raw water.
Owner:HEFEI DEAN WATER TREATMENT EQUIP
Injection molding unit for an injection molding machine
In an injection molding unit for an injection molding machine, two electric drives are provided as electromechanical injection unit and electromechanical dosing unit, the axis of which are aligned with the axis of injection. A compact injection molding unit that is easy to assemble and maintain is achieved due to the fact that the first and second electric drives are disposed on the injection bridge on both sides of a separating plane that extends substantially crosswise to the axis of injection and separates the area of influence of the first electric drive from the area of influence of the second electric drive.
Owner:HEHL KARL
Single-use auto-injector
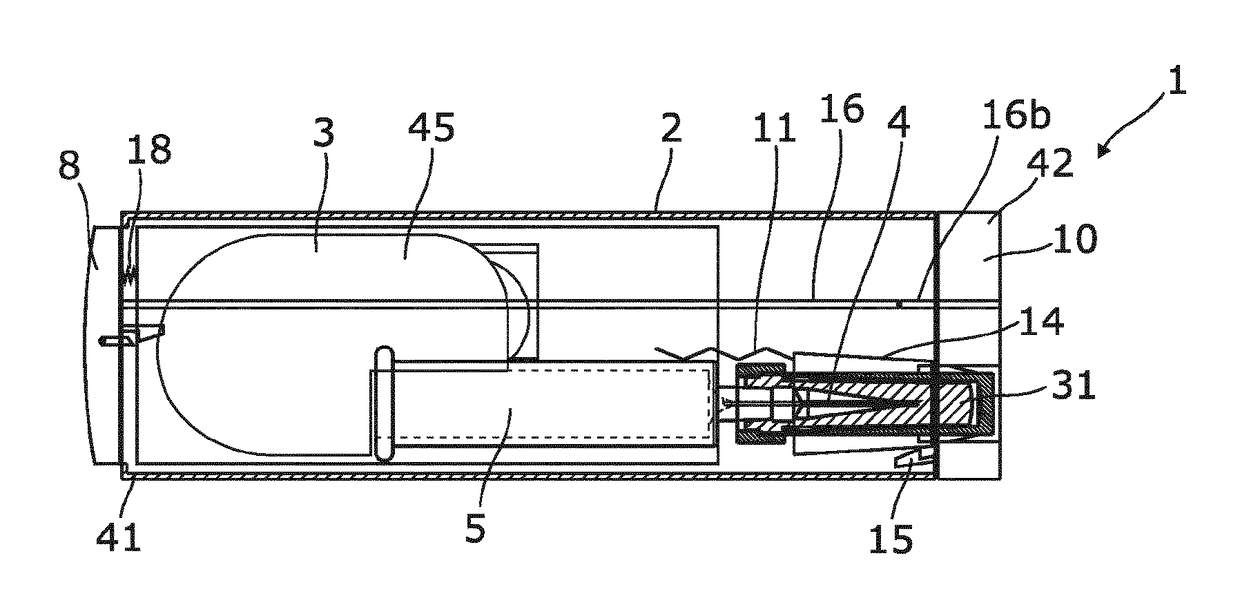
ActiveUS20180304014A1Provide protectionEliminate riskAutomatic syringesPressure infusionBiological activationDosing units
A single-use auto-injector includes a housing and a dosing unit in at least part of the housing. The dosing unit includes a needle, a drug container with drug, a piston movable in the container, a first mechanical power supply for moving the piston to deliver the drug, an activation mechanism, and a mechanical escapement mechanism for controlling the movement of the piston. The auto-injector has a first state in which the needle is protected from needle damage or contamination, a second state in which the needle is ready to penetrate a human body, a third state in which the needle has penetrated the human body and is ready to dose, and a fourth state in which the needle is shielded to avoid unintended needle sticks. A second mechanical power supply is configured to shift state of the auto-injector from the third state to the fourth state.
Owner:AMGEN INC
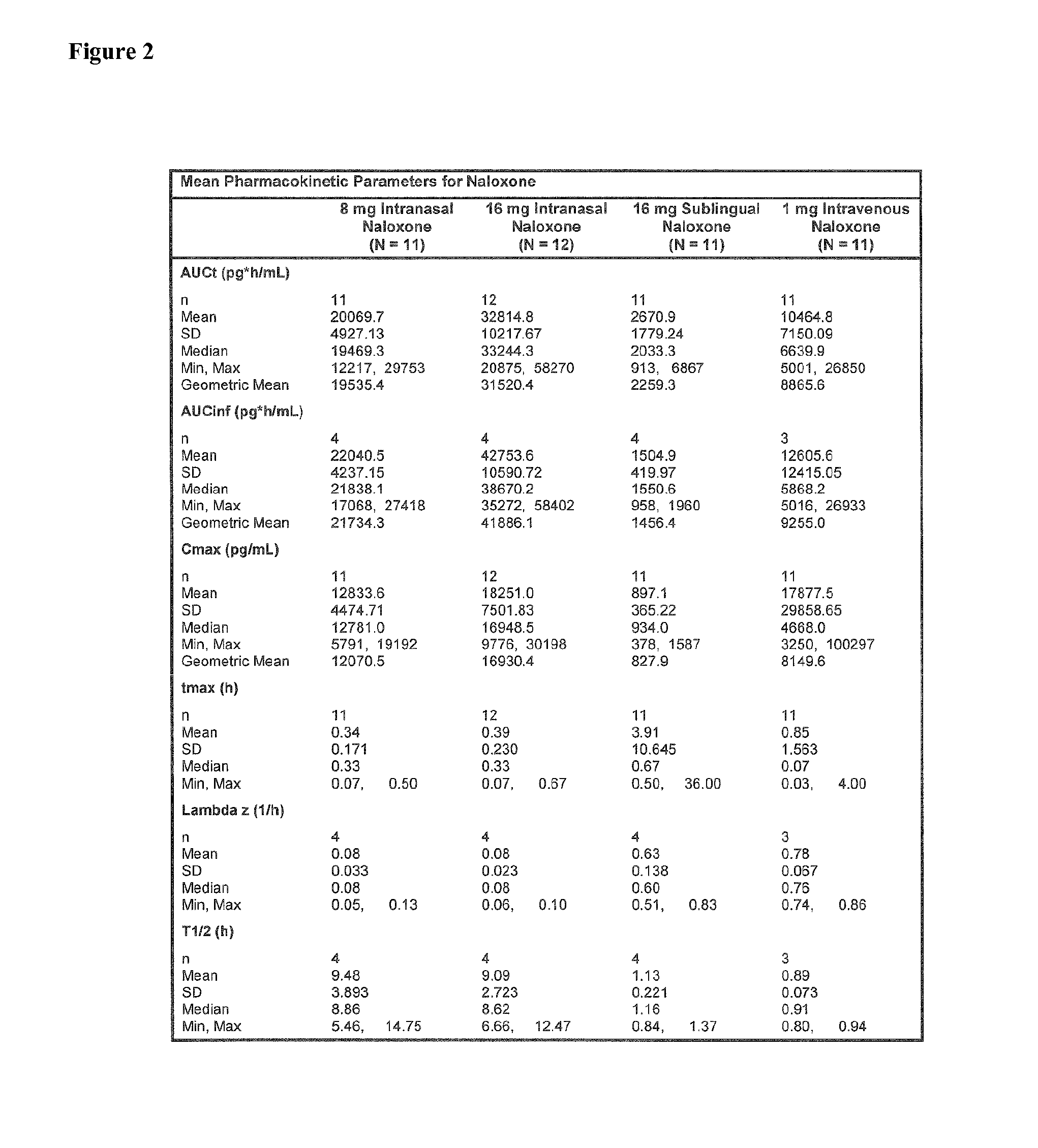
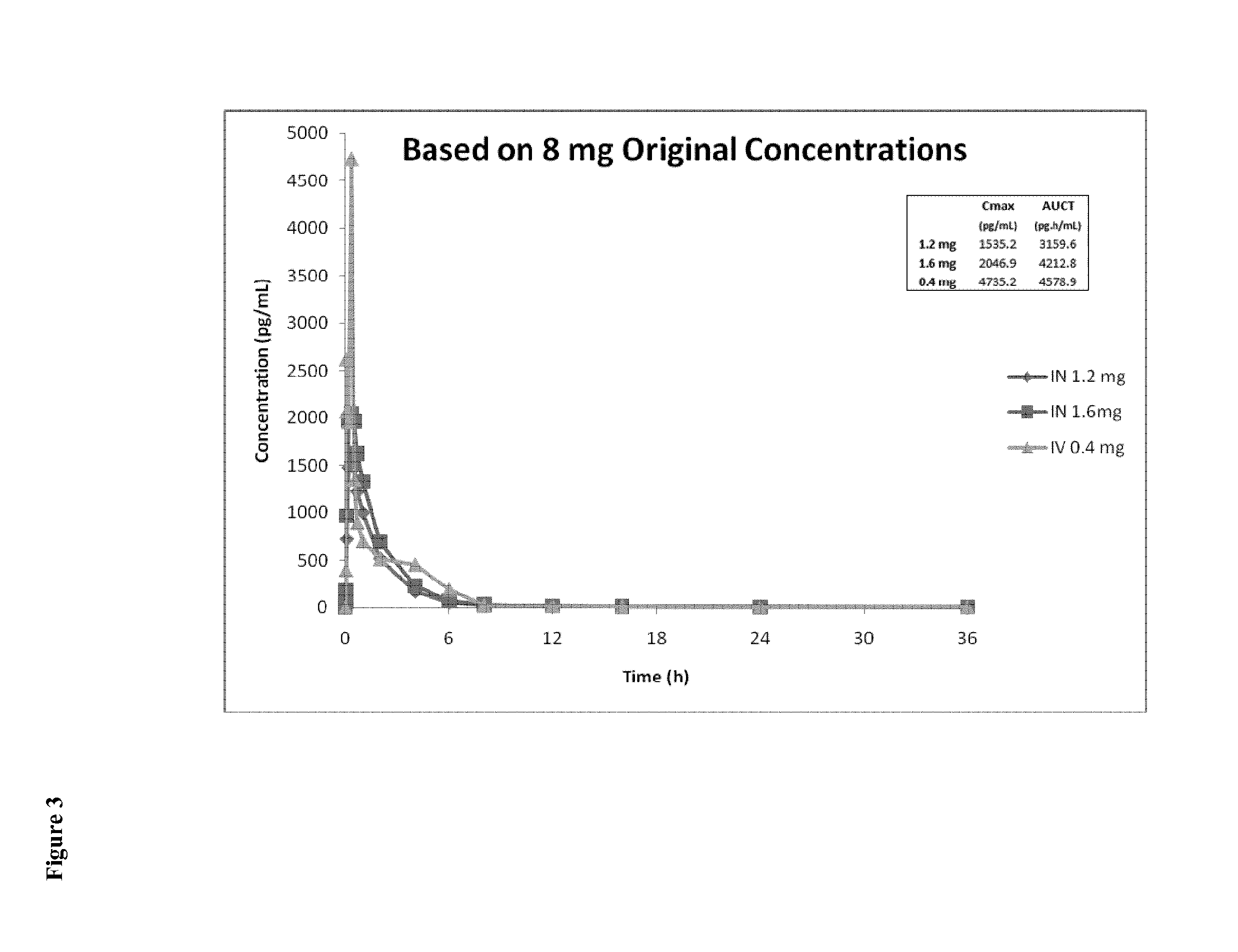
Intranasal Pharmaceutical Dosage Forms Comprising Naloxone
InactiveUS20150018379A1Improve bioavailabilityQuick effectBiocideNervous disorderMedicineOpioid overdose
The present invention relates to an intranasal pharmaceutical dosage form comprising a dosing unit comprising naloxone or a pharmaceutically acceptable salt thereof in an amount of equivalent to ≧0.5 mg naloxone HCl dissolved in an application fluid of a volume of ≦250 μl. Furthermore, the present invention relates to such an intranasal pharmaceutical dosage form for use in the treatment of opioid overdosing and / or at least one symptom thereof.
Owner:EURO-CELTIQUE SA
Method and device for processing polymer-contained waste liquid and fracturing flow-back fluid
ActiveCN104710064ASimple processing methodLow running costMultistage water/sewage treatmentLiquid wasteWater based
The invention discloses a method and a device for processing a polymer-contained waste liquid and a fracturing flow-back fluid. The processing method comprises the following steps: 1) performing ultrasonic ozonization treatment on the polymer-contained waste liquid and the fracturing flow-back fluid; 2) performing catalysis and oxidation treatment on the waste liquid after the ultrasonic ozonization treatment; 3) coagulating, settling and filtering the waste liquid after the catalysis and oxidation treatment to obtain the liquid conforming to the water-based fracturing liquid blending standard and the reinjection water standard. The device mainly comprises an ultrasonic ozonization unit, a catalysis and oxidation unit, a dosing unit and a coagulating-settling-filtering unit, which are sequentially connected. The processing method is simple, the device is practical, the dosing system is relatively safe, so that oil, suspensions and other hazardous substances can be efficiently separated from water under the packless situation, and the waste liquid can be recycled.
Owner:BEIJING OTC ENERGY & ENVIRONMENT ENGINEERING PUBLIC LIMITED COMPANY
Apparatus for dosing liquid gas into a multipane gas unit
Owner:BESTEN EQUIP INC
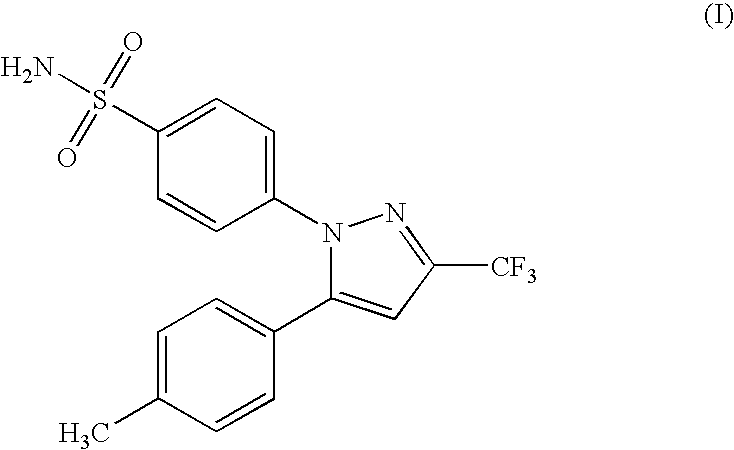
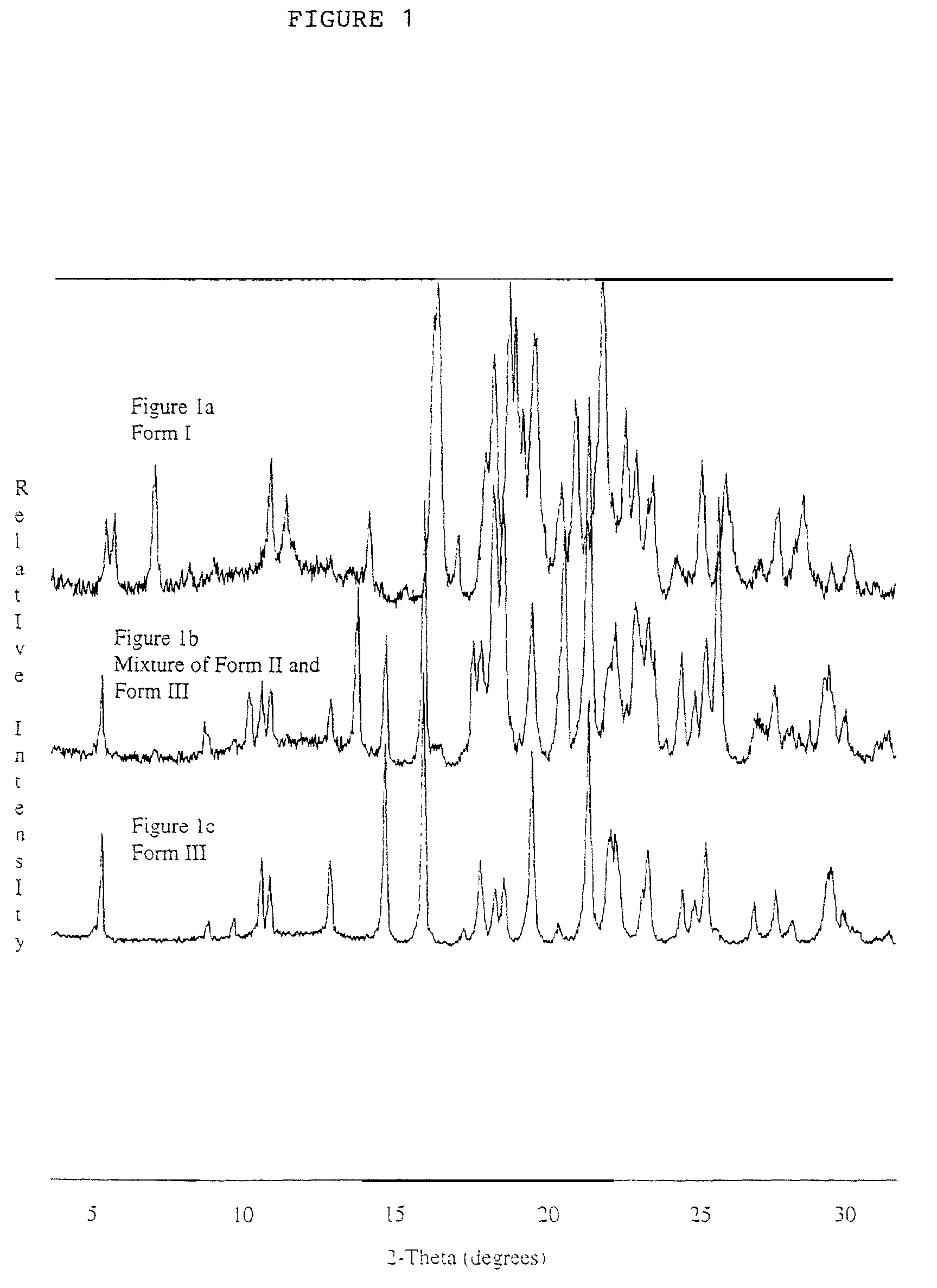
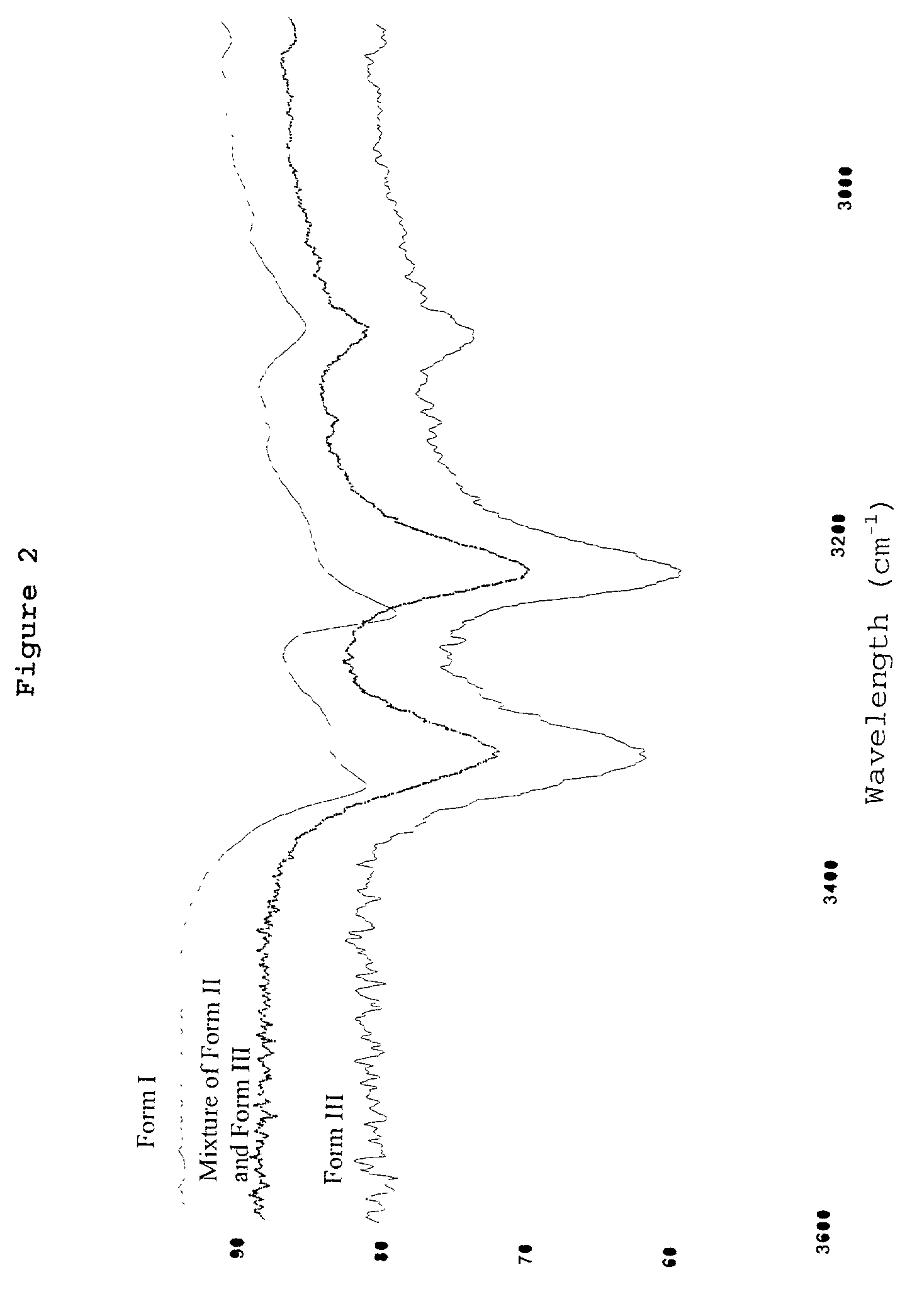
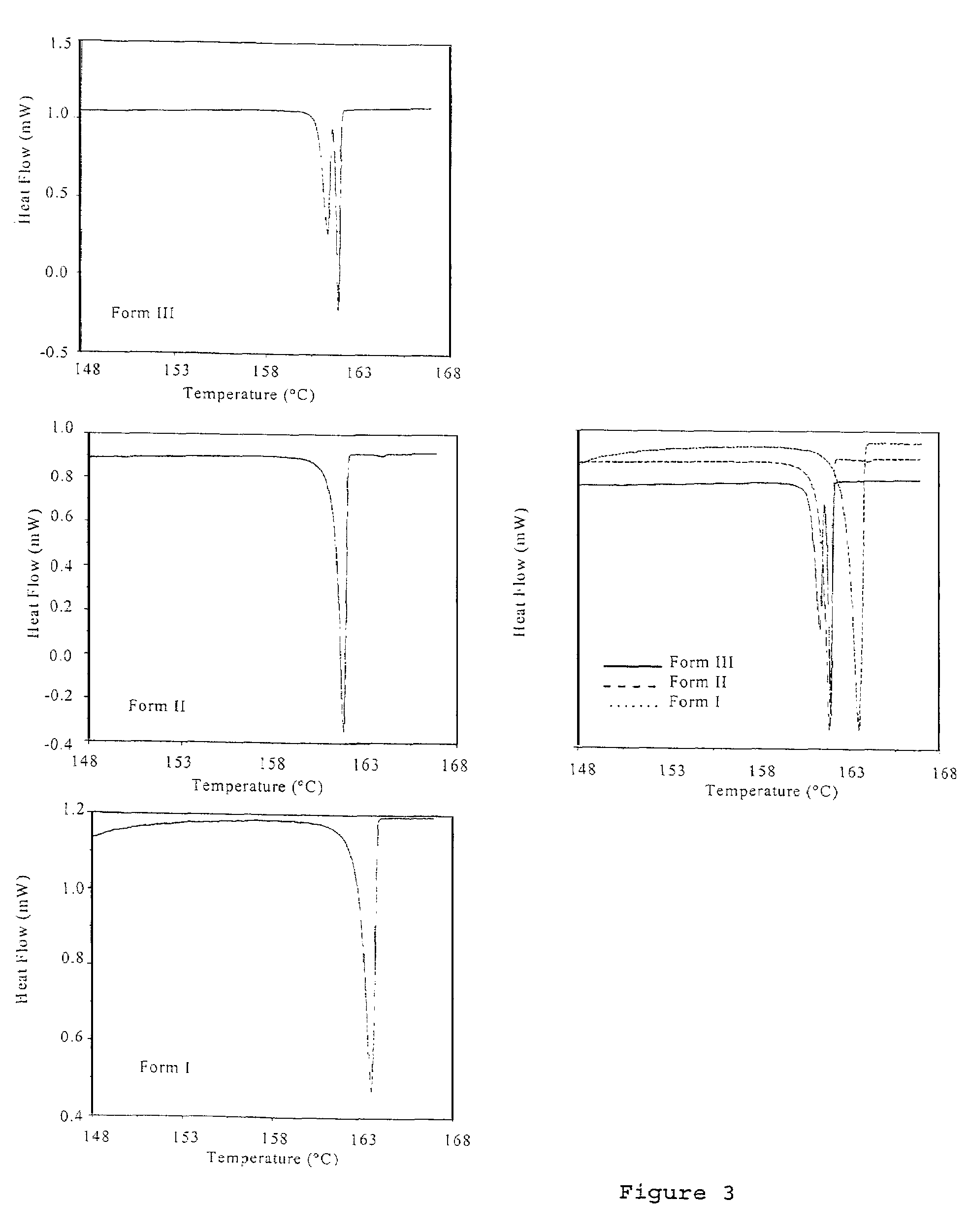
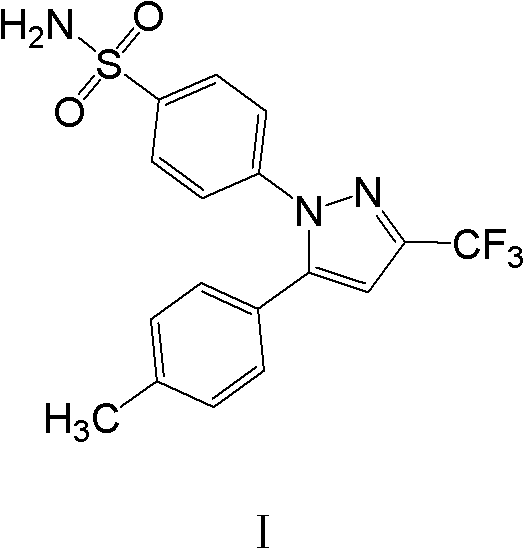
Polymorphic crystalline forms of celecoxib
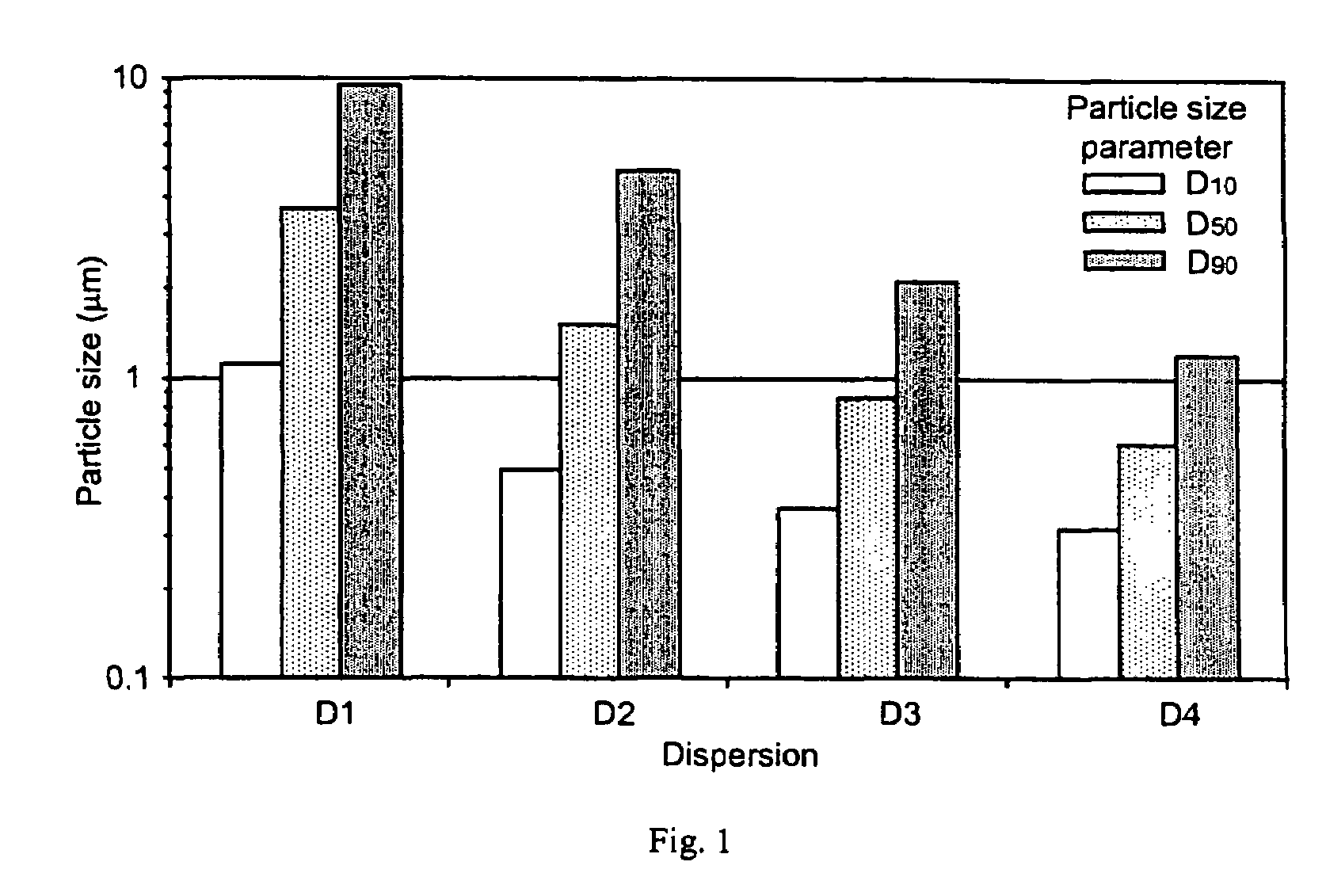
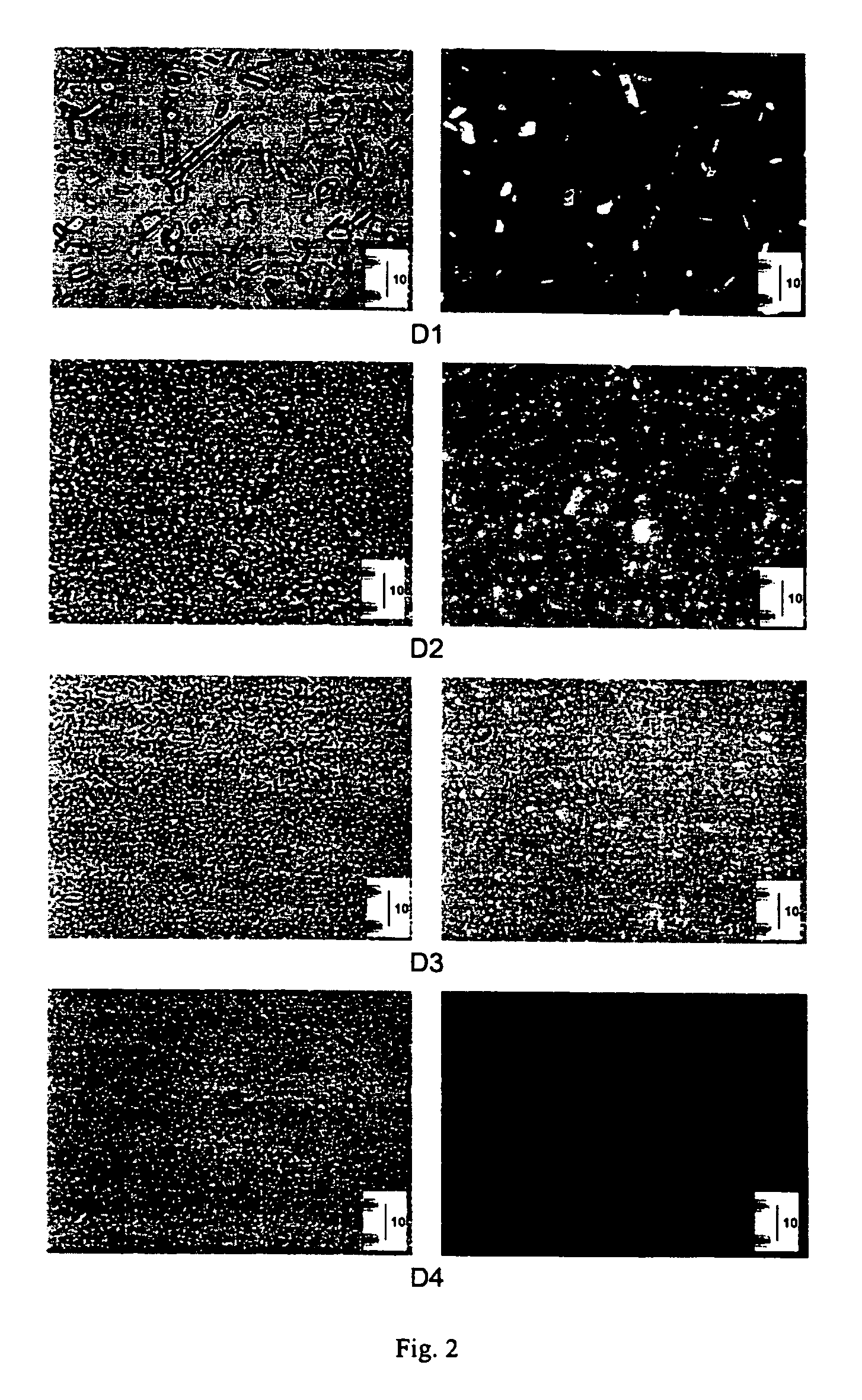
Pharmaceutical compositions are provided comprising one or more orally deliverable dose units, each comprising a selective cyclooxygenase-2 inhibitory compound of low water solubility in a therapeutically effective amount, wherein the compound is present in the form of solid particles, about 25% to 100% by weight of which are smaller than 1 micrometer. The compositions are useful in treatment or prophylaxis of cyclooxygenase-2 mediated conditions and disorders and have particular advantages where rapid onset of therapeutic effect is desired. The novel Form I and Form II crystalline forms of celecoxib are described. The crystalline forms have unique chemical and physical properties relative to other solid state forms of celecoxib and are characterized by their powder x-ray diffraction (PXRD) patterns, differential scanning calorimetric (DSC) thermograms, and other physical characterizations.
Owner:PHARMACIA CORP
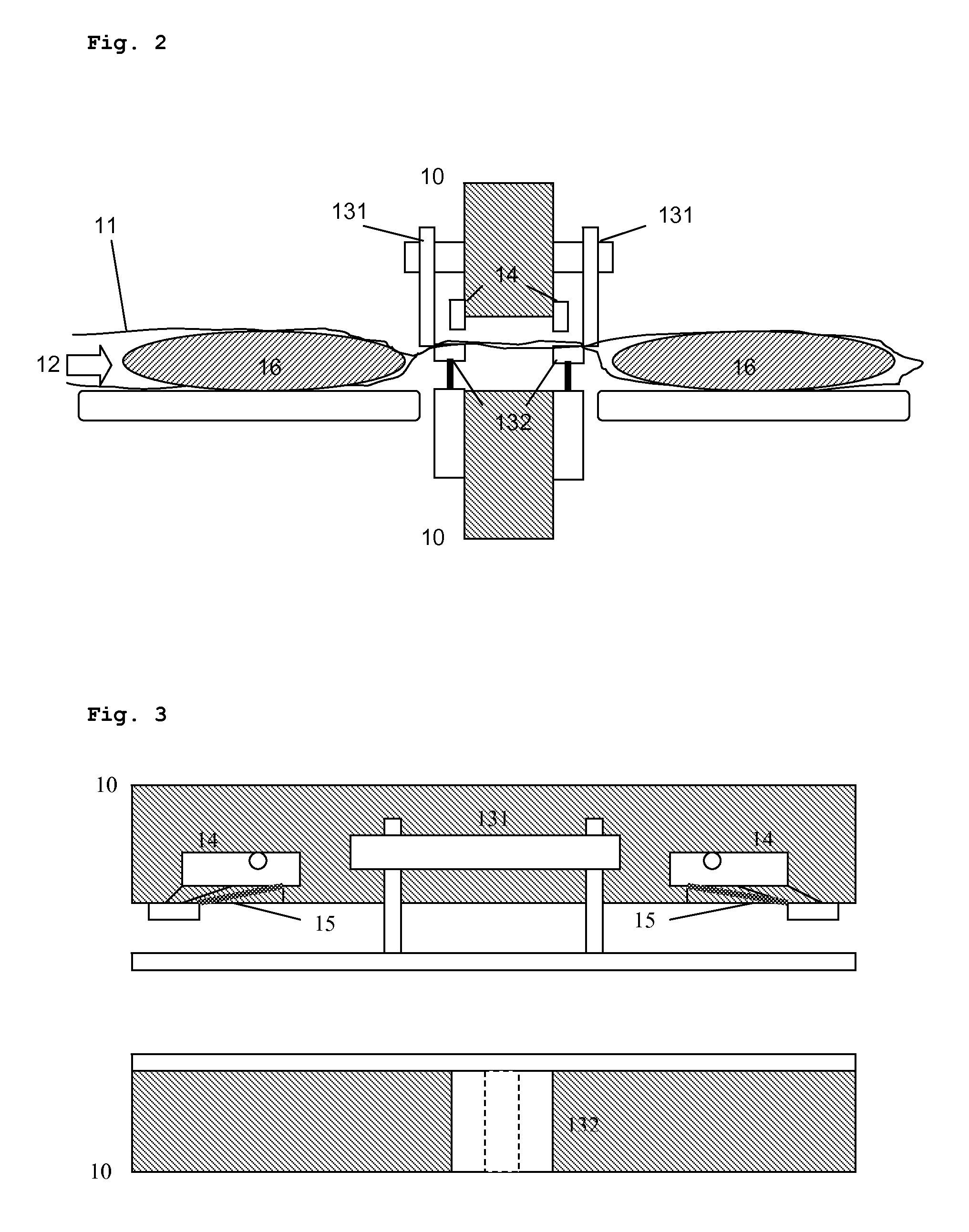
Dosing device for powder dispensers
InactiveCN102293598AWon't get boredDispensing the dose can't go wrongPowdered material dispensingMovable measuring chambersEngineeringMechanical engineering
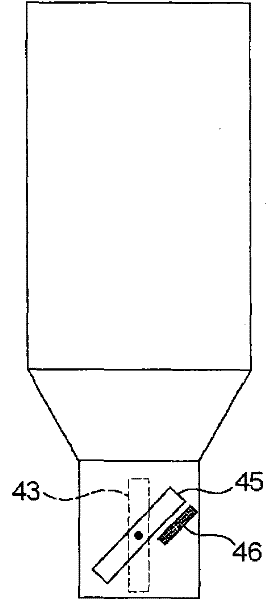
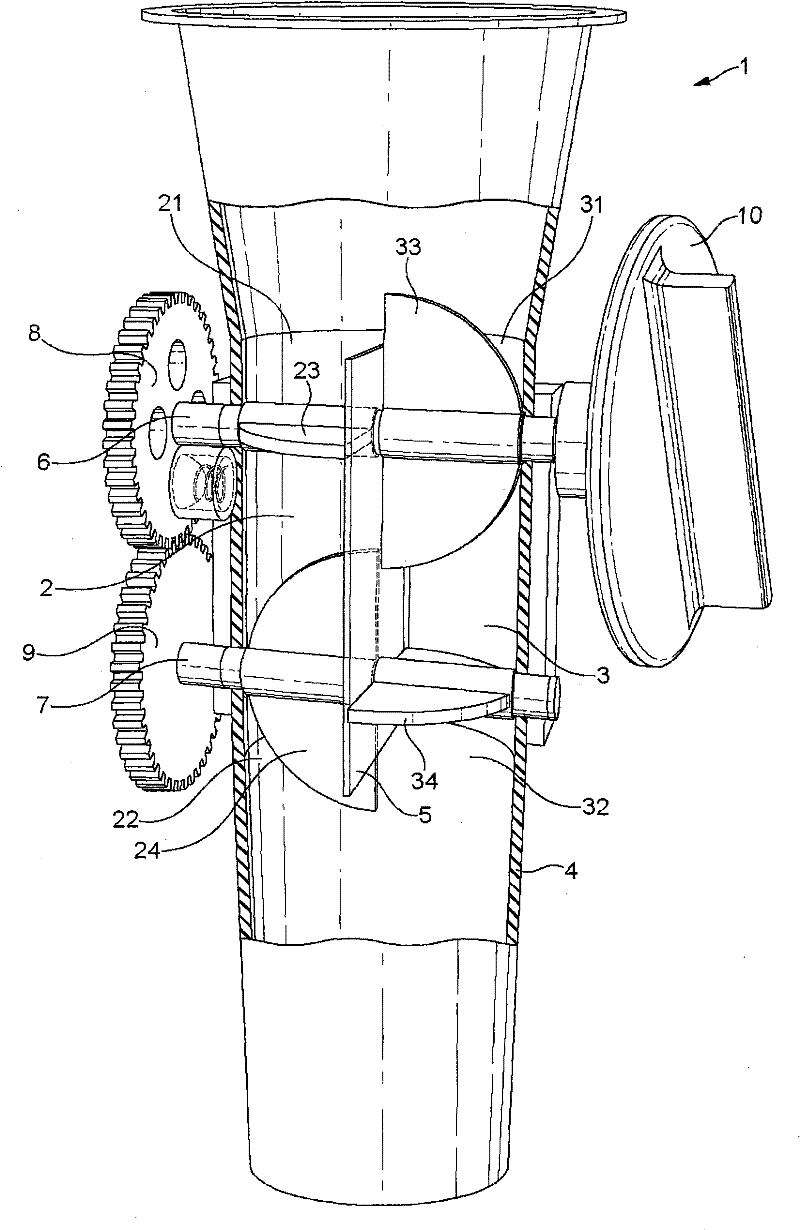
The invention concerns a dosing unit for a powder dispenser comprising two dosing chambers (2,3) each chamber comprising: one upstream inlet (21, 31) and one downstream outlet (22, 32), an upstream means (23, 33) for opening and closing the inlet, a downstream means (24, 34) for opening and closing the outlet, the upstream means (23, 33) closing the inlet (21, 31) when the downstream means (24, 34) opens the outlet (22, 32) and reciprocally, wherein the upstream means (23) of the first chamber closes the first chamber (2) when the upstream means (33) of the second chamber opens the second chamber (3) and reciprocally.
Owner:SOC DES PROD NESTLE SA
Packaging system for detergents or cleansers
InactiveUS20080261851A1Good storage stabilityMaintain stabilityDetergent materialsPackagingCleansers skinWater insoluble
Packaging systems for detergent or cleanser dosing units comprising: a) a primary packaging system in the form of a number (n)>2 of water-insoluble bags, each of these bags containing a number (x)>2 of detergent or cleanser dosing units, and; b) a secondary packaging system in the form of a water-insoluble bag that contains the (n) water-insoluble bags of the first packaging system. The inventive packaging systems are suited for increasing the stability of the detergent or cleanser dosing units contained therein.
Owner:HENKEL KGAA
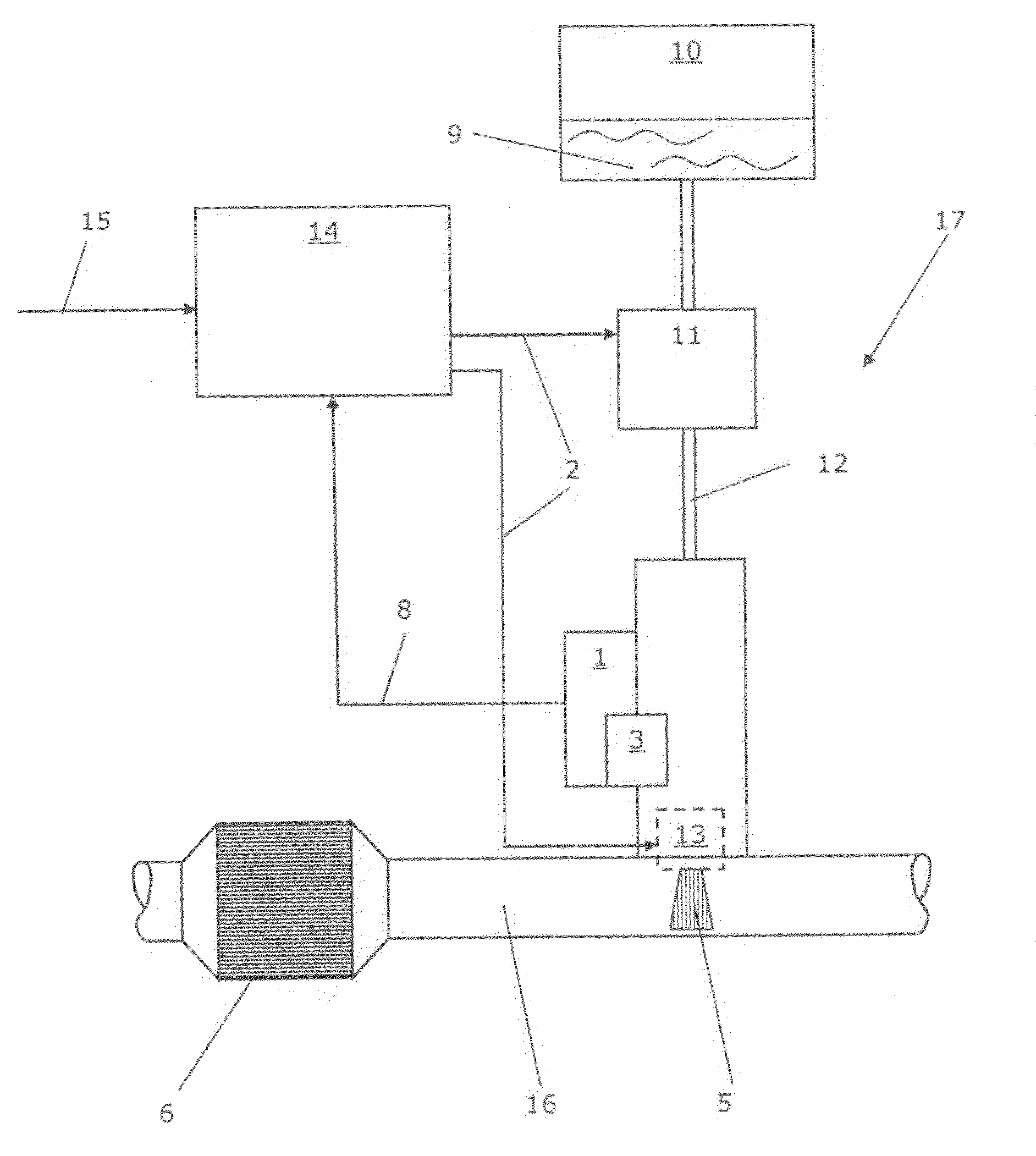
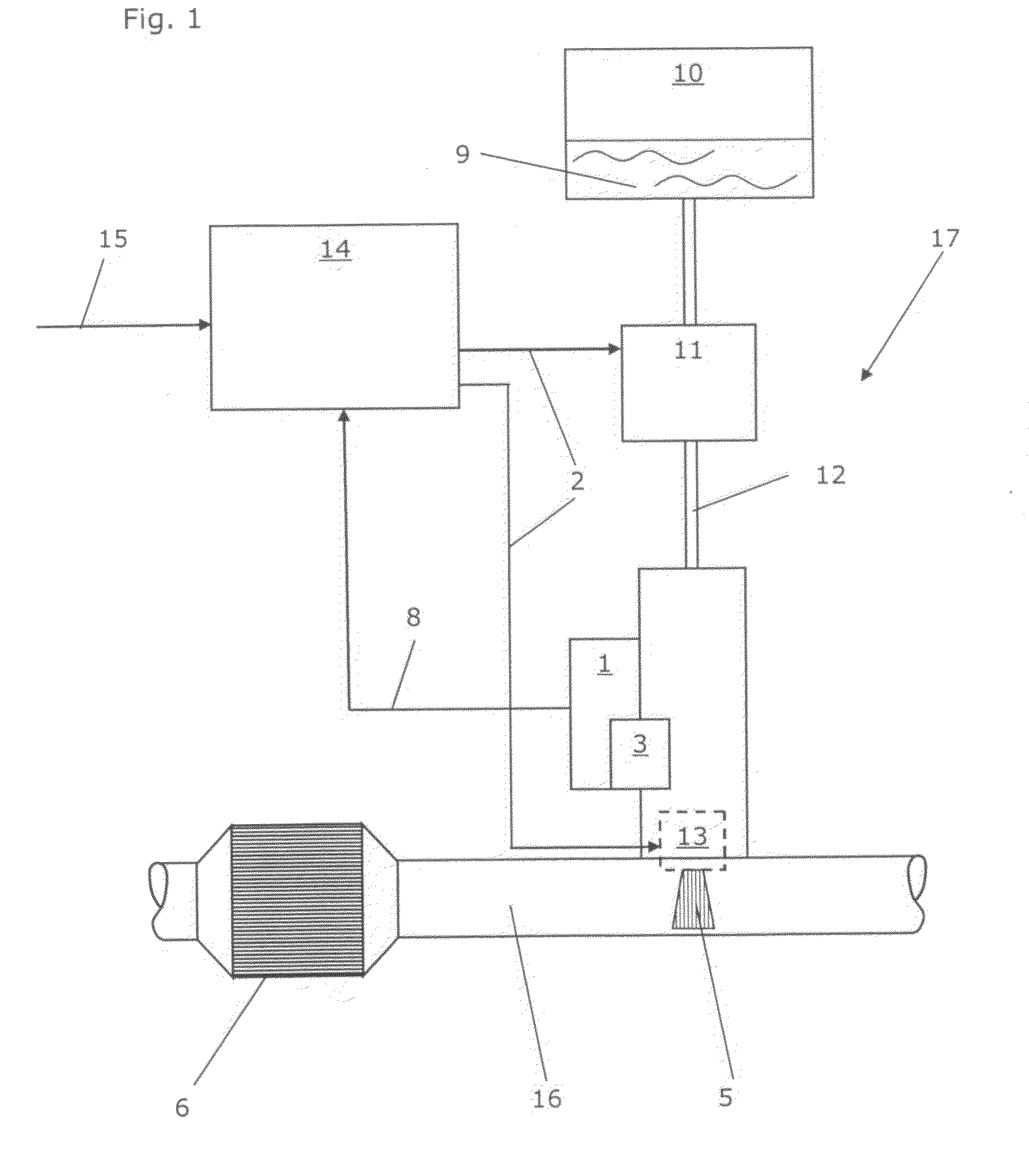
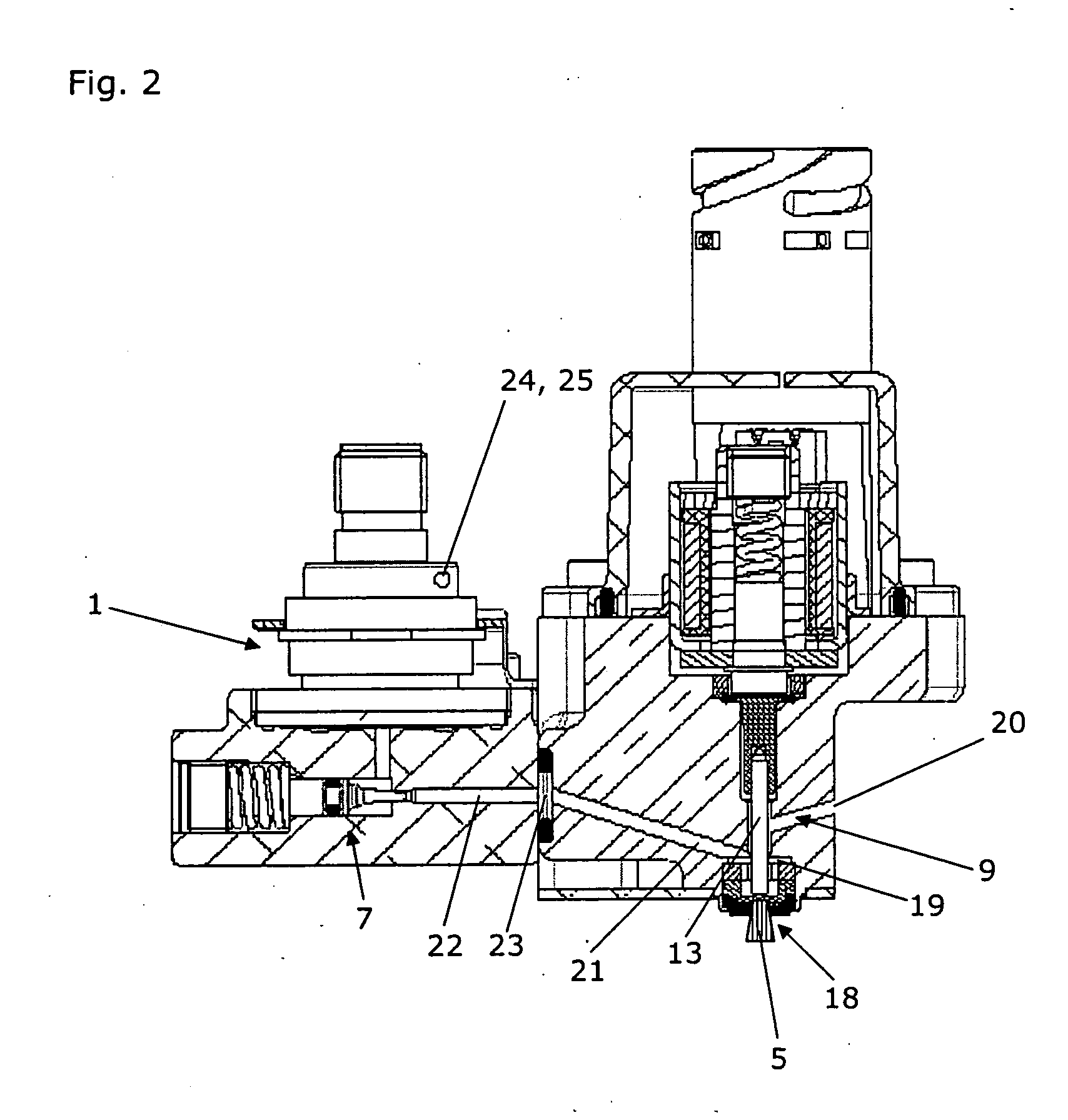
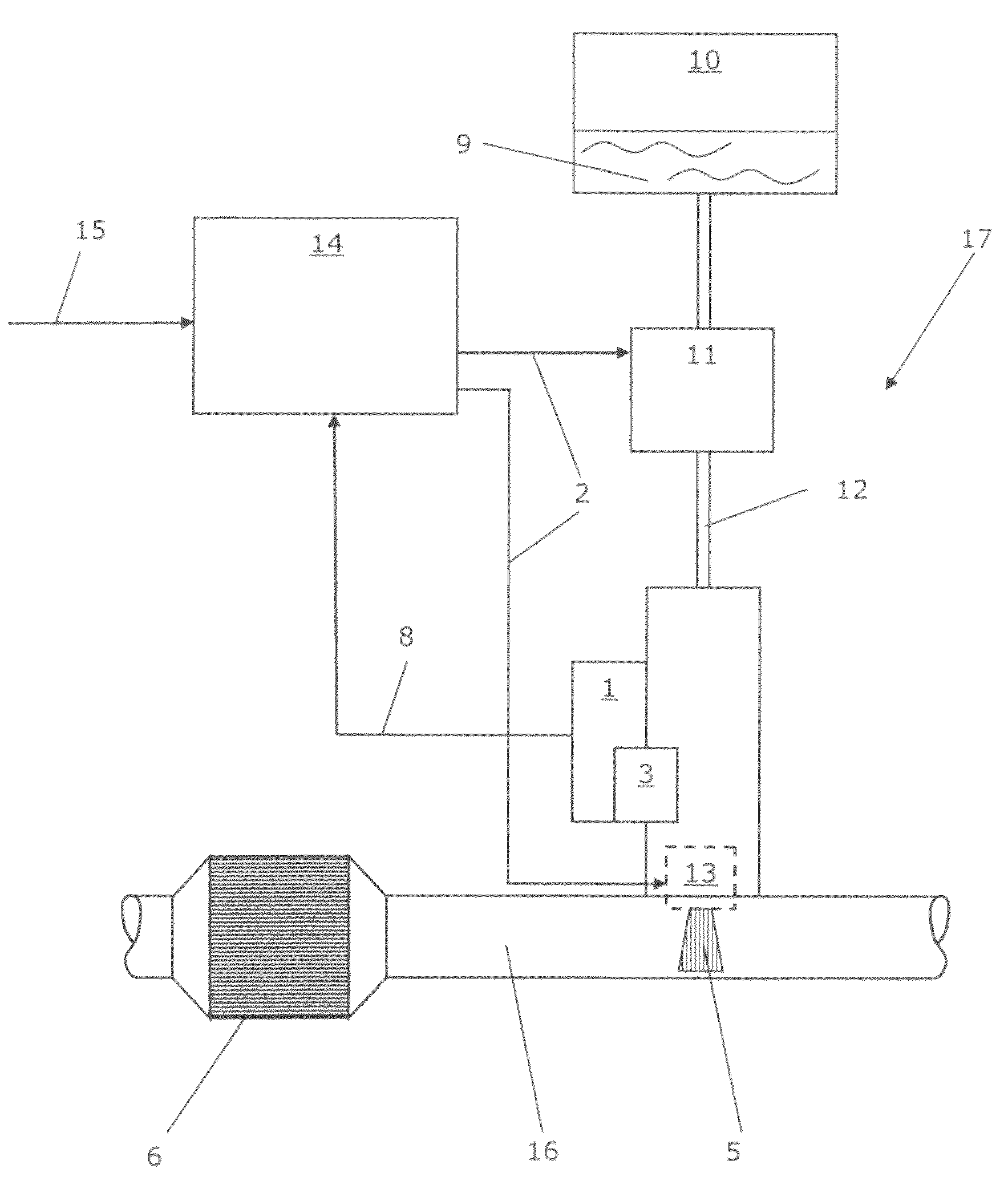
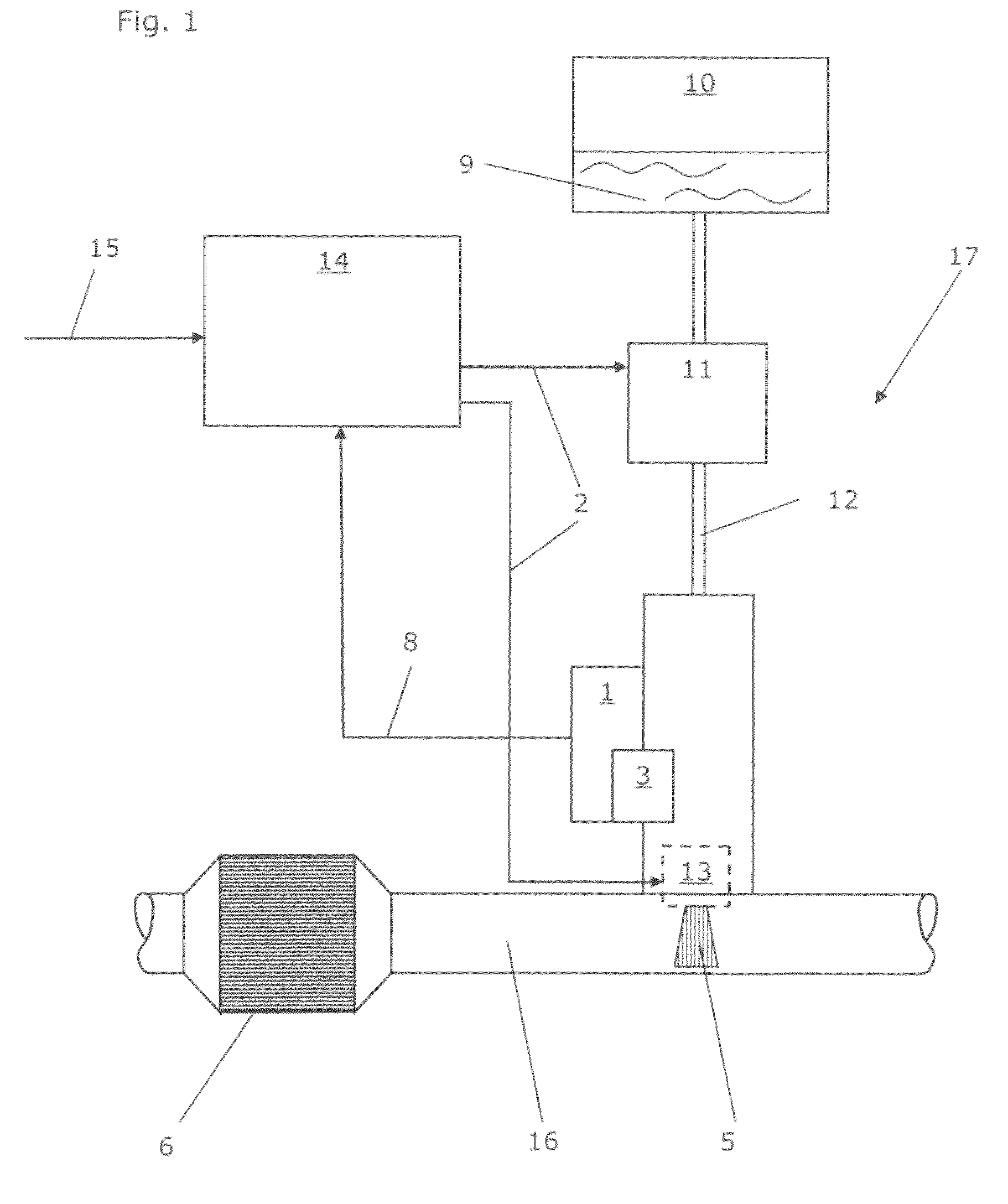
Calibrated dosing unit, especially of an exhaust gas treatment unit
ActiveUS20080178580A1Reduce complexityTesting/calibration apparatusInternal combustion piston enginesElectricityTransducer
The invention relates to a volume quantity dispensing unit, in particular as a unit for metering an aqueous solution such as a urea / water solution, which can be used in an exhaust gas aftertreatment unit (17), comprising a pressure transducer (1), in particular comprising an electric pressure sensor, the volume quantity dispensing of the volume quantity dispensing unit following an electric signal (2) and the volume quantity dispensing unit being calibrated. There is provided at least one means (3) for changing a pressure value (4), which means changes the pressure value (4) in such a way that the pressure value (4), which is intended to correspond to the pressure in the volume quantity dispensing unit, is an input signal for a volume quantity (5) to be dispensed.
Owner:CUMMINS LTD
Celecoxib composition, and preparation method and use thereof
The invention provides a celecoxib composition. The celecoxib composition contains one or more types of dose units which can be orally released, and each of the dose units contains 50-500mg of celecoxib D95 particles and one or more types of mixtures of pharmaceutical excipients. The composition can be used for treating or preventing diseases caused by COX-2.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Calibrated dosing unit, especially of an exhaust gas treatment unit
ActiveUS8266892B2Reduce complexityInternal combustion piston enginesExhaust apparatusElectricityTransducer
The invention relates to a volume quantity dispensing unit, in particular as a unit for metering an aqueous solution such as a urea / water solution, which can be used in an exhaust gas aftertreatment unit (17), comprising a pressure transducer (1), in particular comprising an electric pressure sensor, the volume quantity dispensing of the volume quantity dispensing unit following an electric signal (2) and the volume quantity dispensing unit being calibrated. There is provided at least one means (3) for changing a pressure value (4), which means changes the pressure value (4) in such a way that the pressure value (4), which is intended to correspond to the pressure in the volume quantity dispensing unit, is an input signal for a volume quantity (5) to be dispensed.
Owner:CUMMINS LTD
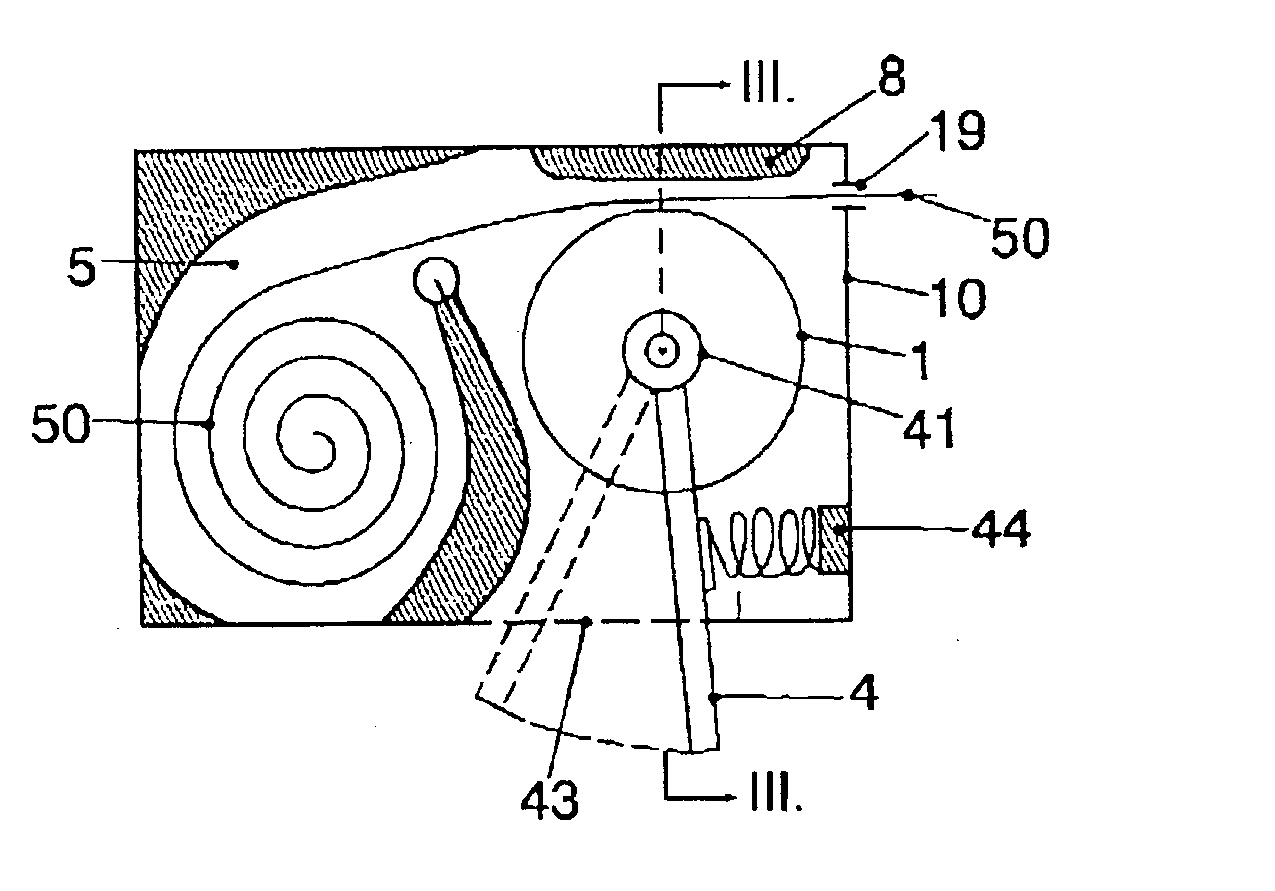
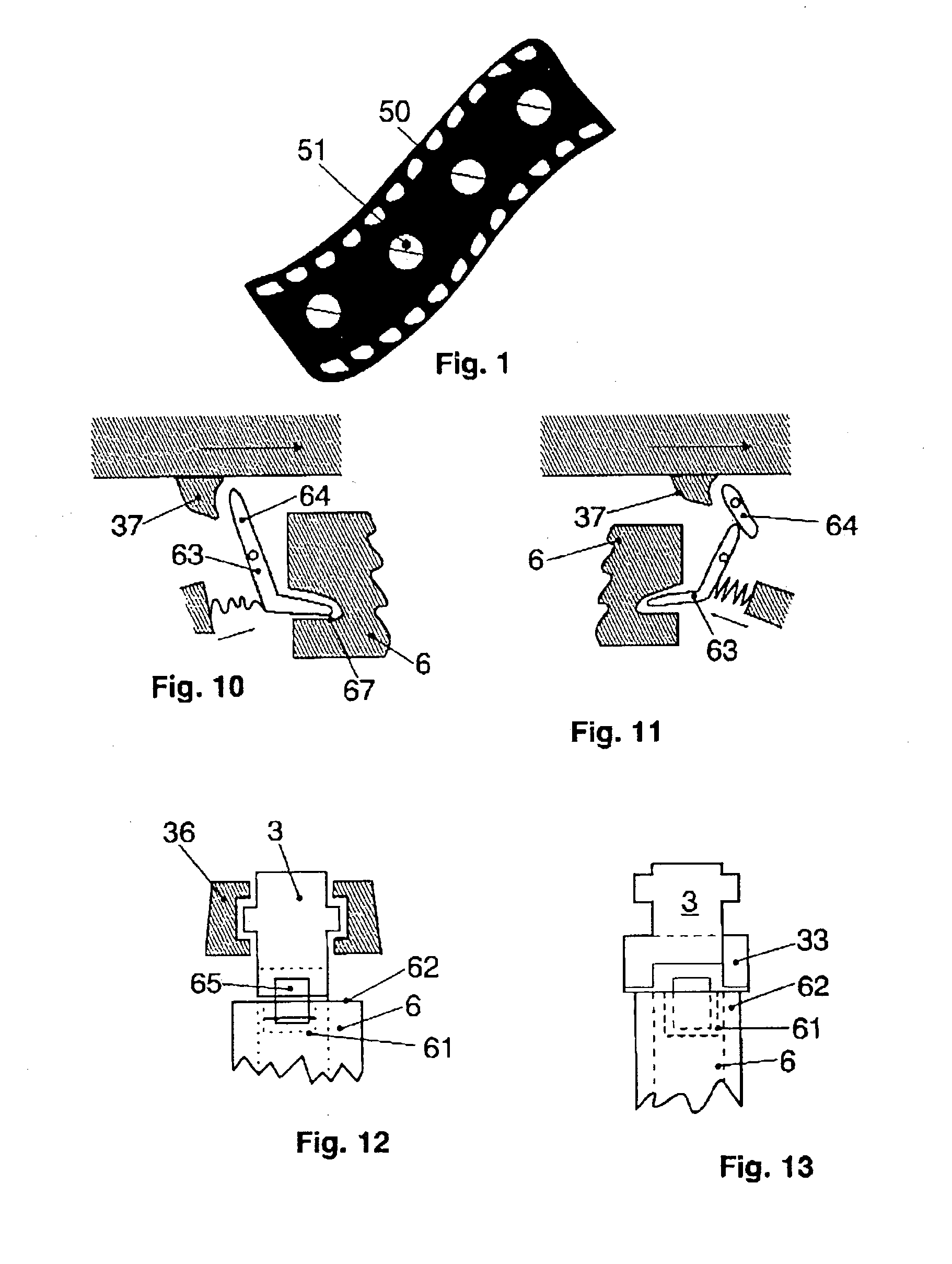
Apparatus for dosage of medicaments
InactiveUS20030102324A1Counter becomes reusableEasy assessment processCoin-freed apparatus detailsPharmaceutical containersSubject matterBiomedical engineering
The subject matter of the invention is an apparatus to dose medicaments and control taking in the medicaments, which apparatus consists of a storage unit (5) to store tablets or capsules, a dosing unit and a counter (7). The characteristic feature of the apparatus according to invention is that the medicaments (like tablets or capsules) (51) are packed in a medicament tape (50) and stored in the storage unit (5) and cylinders (1) furthering said medicament tape (50) are attached to the storage unit (5) and the said cylinders are mechanically coupled with a manual driving unit (4).
Owner:ILLYES MIKLOS
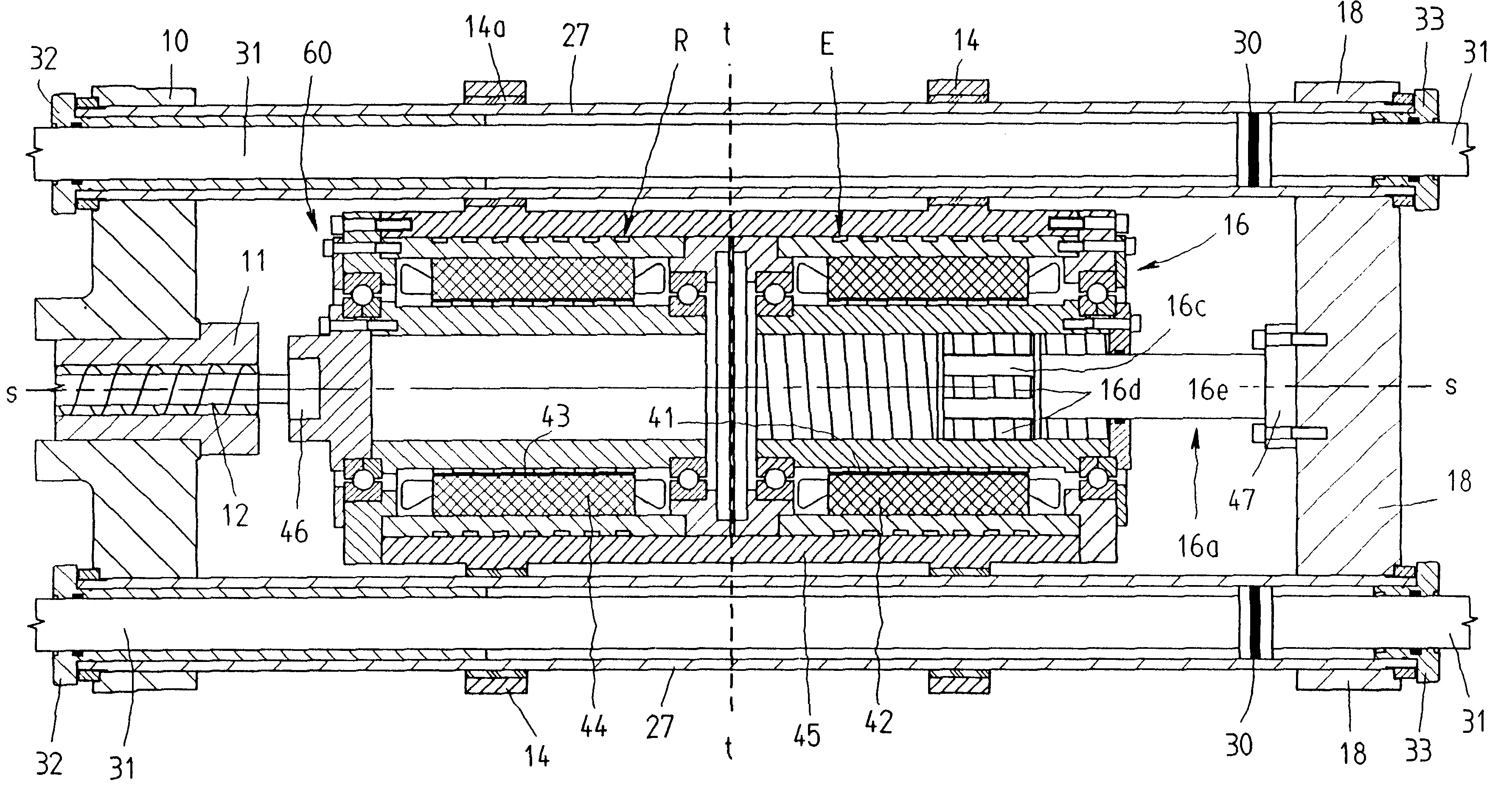
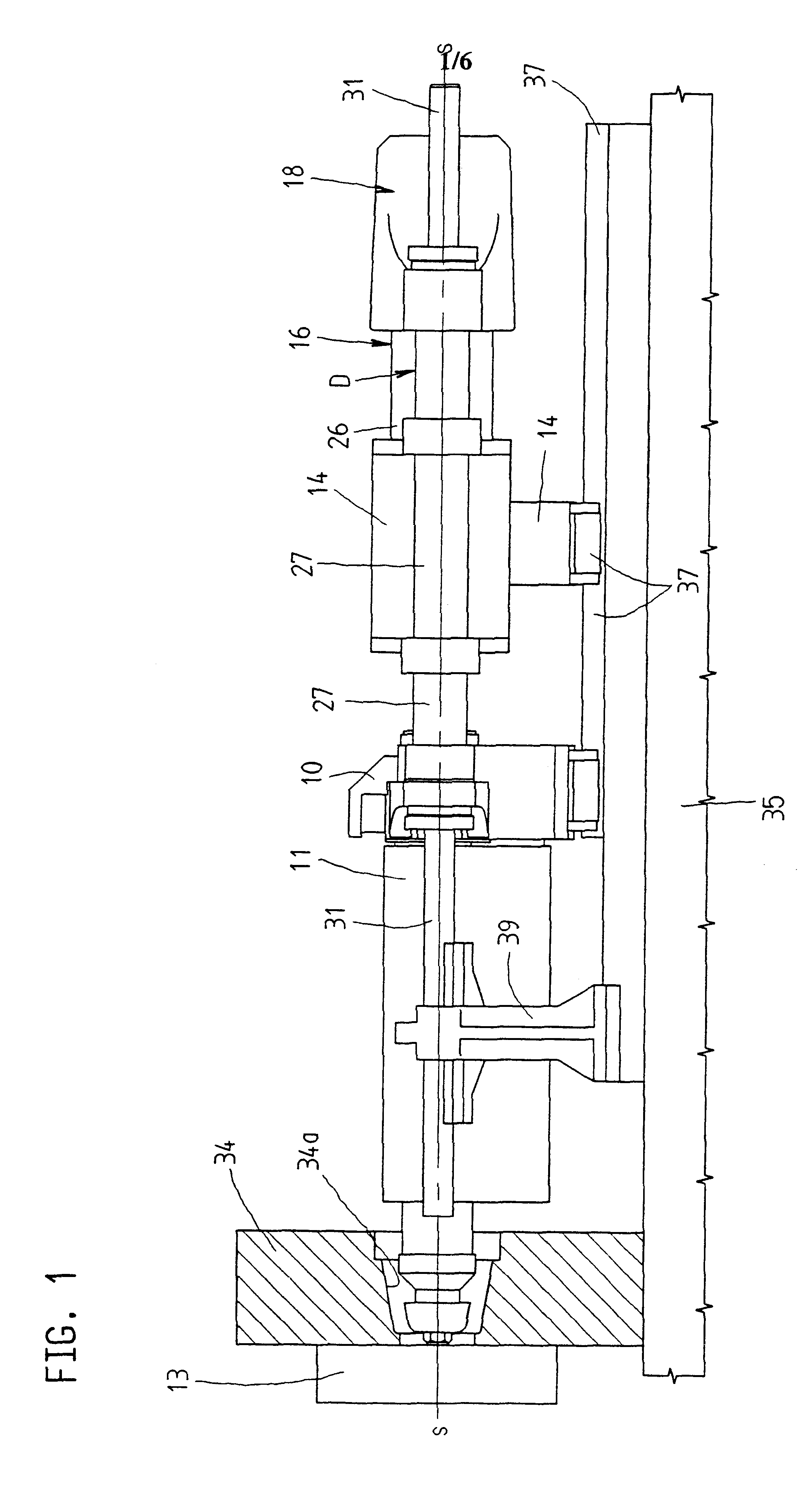
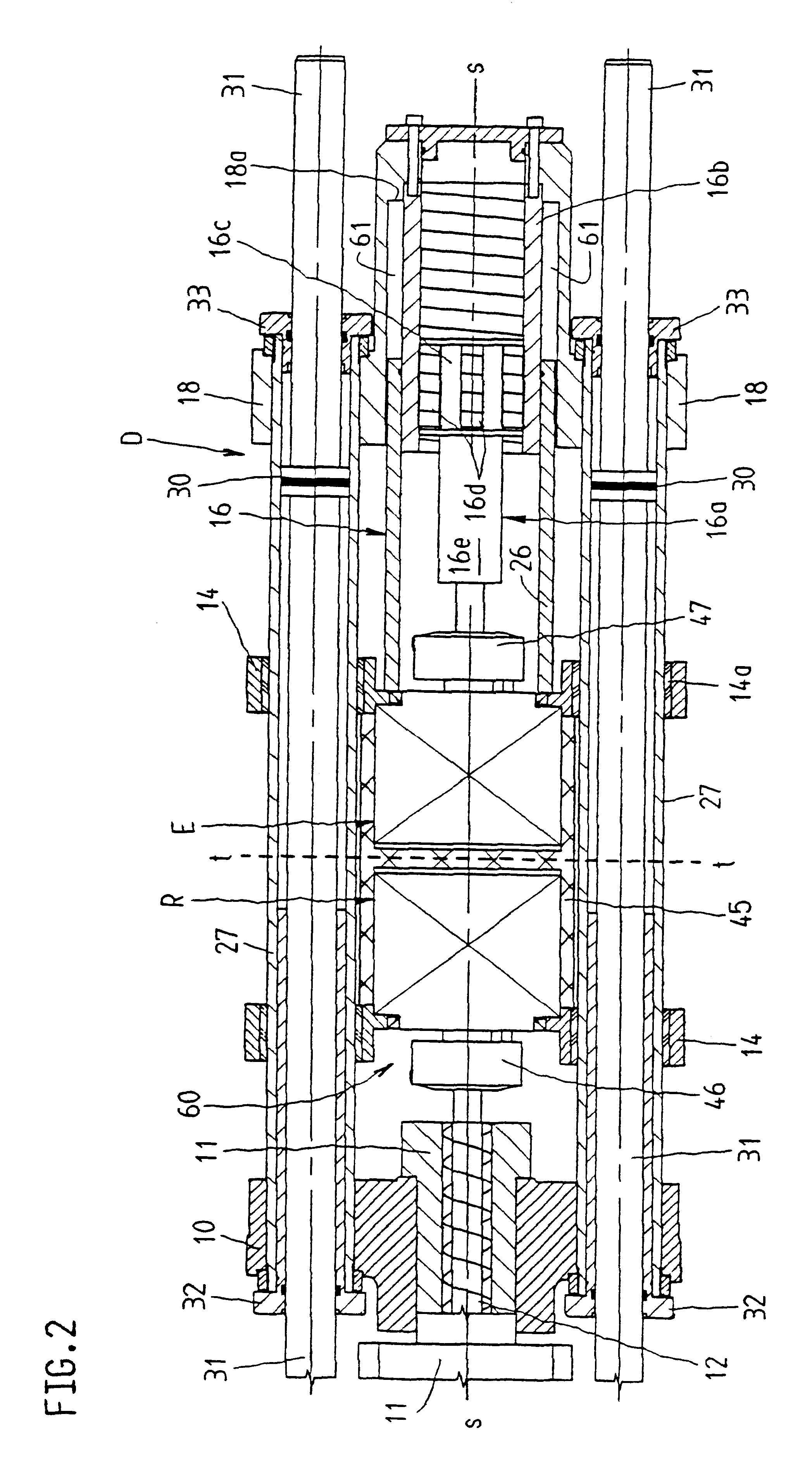
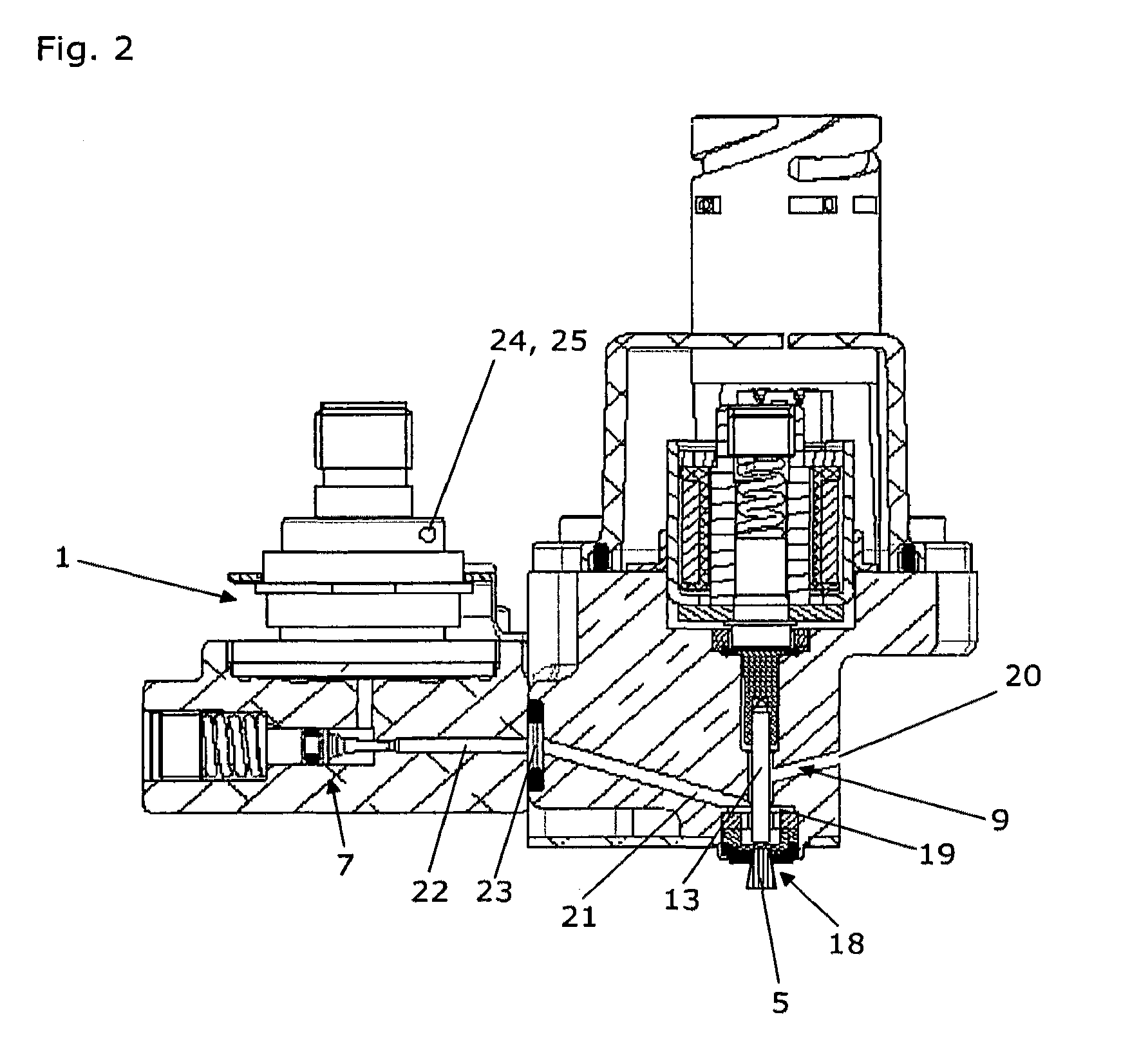
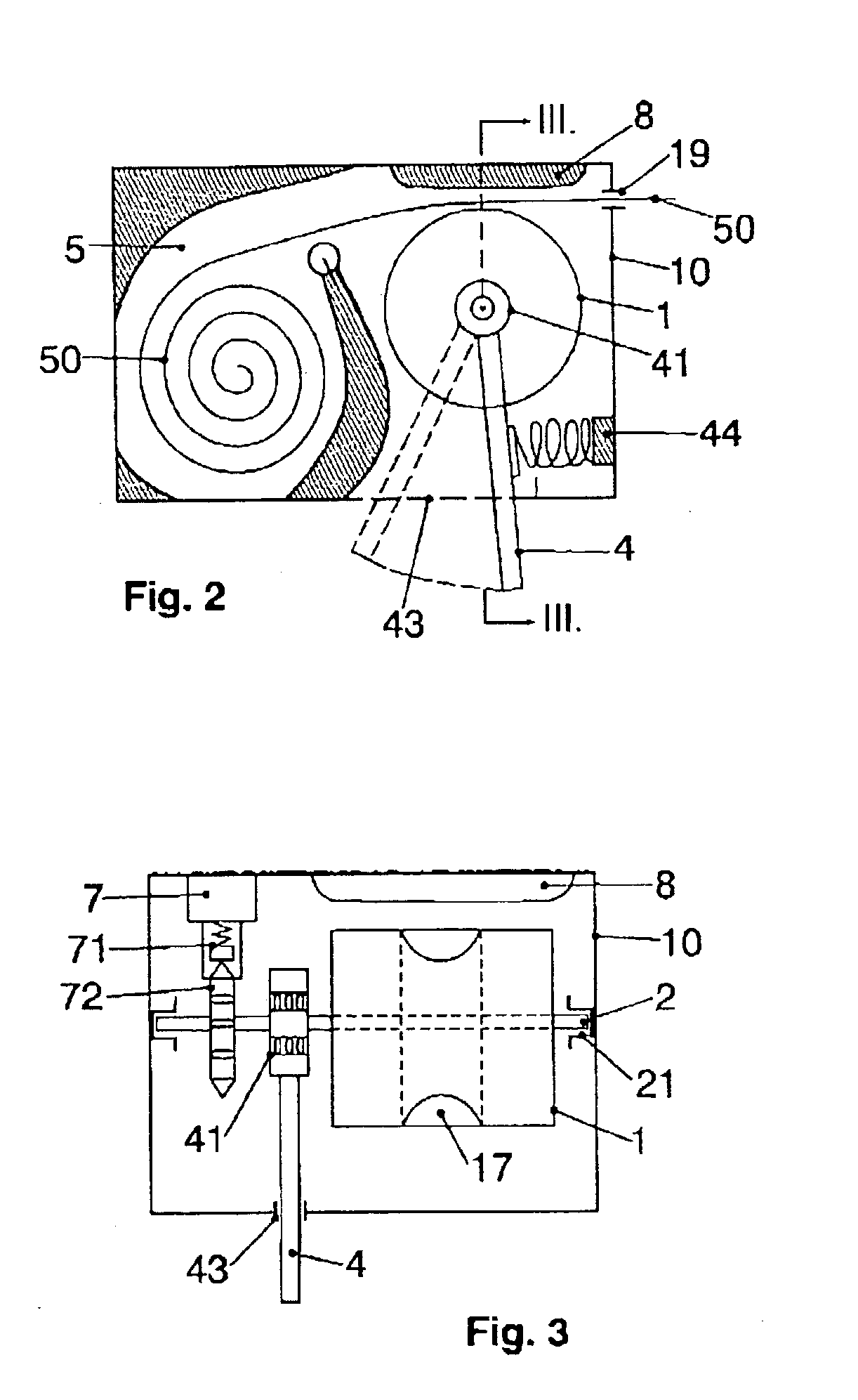
Infusion pump device with cylinder-piston dosing unit and optical piston position detection
ActiveCN103458940AMonitor verticalMonitoring and/or rotational displacementMedical devicesPressure infusionEngineeringMechanical engineering
A dosing unit (12) for an ambulatory infusion pump device (10) comprises a cylinder pump with a cylinder (14) and a piston (16) arranged in said cylinder. The piston has a shaft (18) with a first threaded segment (50) interacting with a threaded portion (54) of the cylinder, and can be displaced along a longitudinal axis (48) of the cylinder by rotating the piston in regard to the cylinder around said axis. Furthermore the piston comprises means that allow the relative or absolute determination of the longitudinal and / or rotational displacement of the piston in regard to the cylinder. In a preferred embodiment the piston shaft (18) comprises a second segment (51) provided with optically detectable markings (64, 65) that allow the monitoring of the longitudinal and / or rotational displacement of the piston in regard to the cylinder.
Owner:F HOFFMANN LA ROCHE & CO AG
Apparatus and method for preparing and dispensing a single dose of a food product and a relative single-dose unit
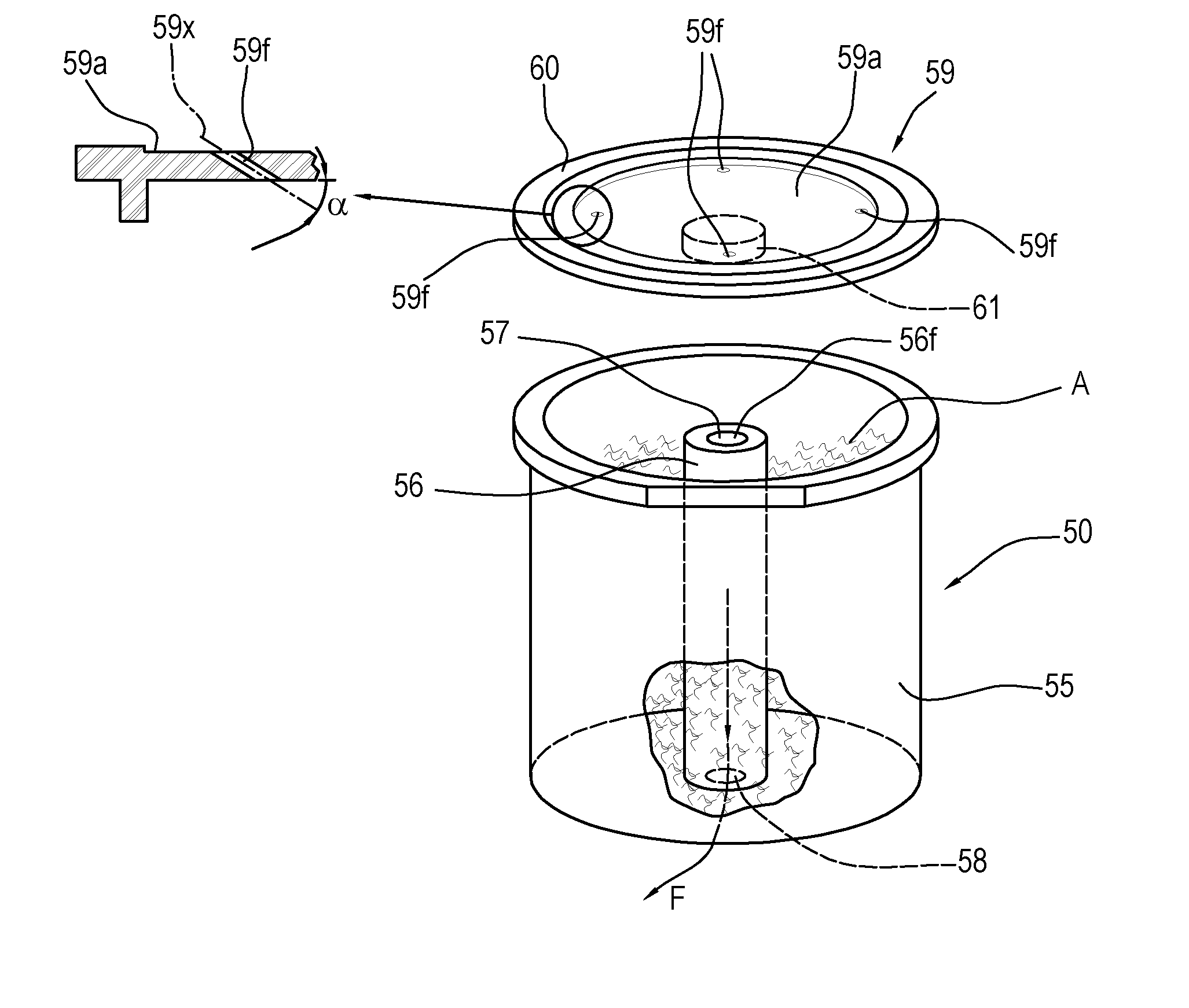
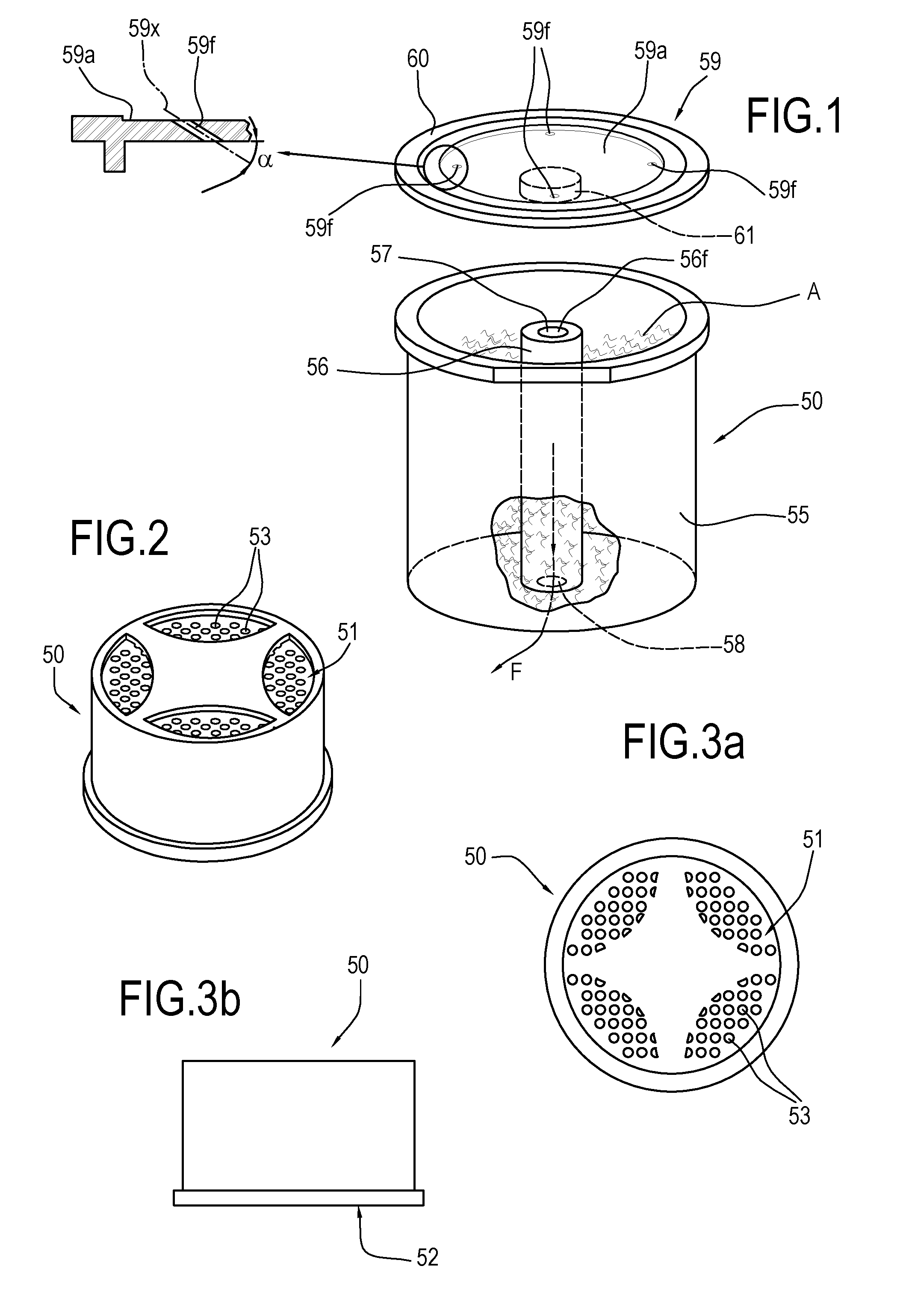
ActiveUS20160316781A1Low production costHealth and safetyFrozen sweetsBeverage vesselsEngineeringSolvent
Described is an apparatus for preparing and dispensing a single dose of a food product comprising:—means (2) for feeding a solvent liquid having at least one duct (28) for dispensing the liquid;—a cavity (11) for housing a single-dose unit (50) of a food product comprising an inlet connected to the duct (28) for dispensing the liquid designed for feeding the liquid to the single-dose unit (50); the cavity having at least one duct (8) for transferring the product-liquid mixture;—a unit or chamber (9) for cooling the mixture fed by the transfer duct (8) inside the cooling unit;—a unit (14) for dispensing the product formed inside the cooling unit (9) connected to the same unit, for dispensing the product formed in a single dose.
Owner:RDL
Cyclooxygenase-2 inhibitor compositions having rapid onset of therapeutic effect
Pharmaceutical compositions are provided comprising one or more orally deliverable dose units, each comprising a selective cyclooxygenase-2 inhibitory drug of low water solubility in a therapeutically effective amount, wherein the drug is present in the form of solid particle, about 25% to 100% by weight of which are smaller than 1 μm. The compositions are useful in treatment or prophylaxis of cyclooxygenase-2 mediated conditions and disorders and have particular advantages where rapid onset of therapeutic effect is desired.
Owner:PHARMACIA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com