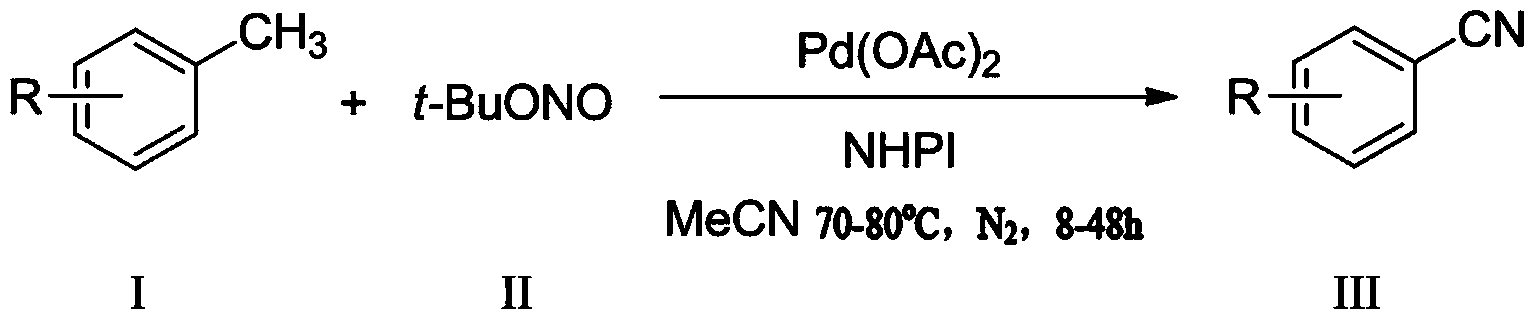Preparation method for aromatic nitrile compound
A compound, aromatic nitrile technology, applied in the field of preparation of aromatic nitrile compounds, can solve the problems of poor functional group compatibility, small range of reaction substrates, harsh reaction conditions, etc., to achieve wide substrate universality and easy purification of products , the effect of simple substrate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
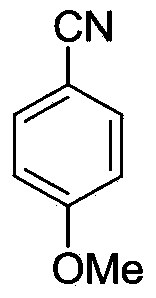
[0029] Synthesis of p-methoxybenzonitrile
[0030] Under the protection of nitrogen, add 61mg (ie 0.5mmol) p-methoxytoluene, 103mg (ie 1.0mmol) nitroso tert-butyl ester, 25mg (0.15mmol) N-hydroxyphthaloyl to the microwave tube Amine and 5.6 mg of palladium acetate (ie 0.025 mmol) were heated to 70° C. in 0.5 mL of acetonitrile to react for 24 hours. After the reaction system was cooled to room temperature, 2 mL of dichloromethane was added for dilution. The mixture was filtered and concentrated. The reaction solution is purified by column chromatography with petroleum ether as an eluent to obtain p-methoxybenzonitrile, and its structure is shown in the following formula:
[0031]
[0032] The compound is a white solid with a yield of 84%, and its NMR data are as follows:
[0033] 1 HNMR(CDCl3,400MHz):δ=7.59(d,J=8.8Hz,2H),6.97(d,J=8.8Hz,2H),3.86(s,3H); 13 CNMR(CDCl3,100MHz):δ=162.8,133.9,119.2,114.7,103.9,55.5.
Embodiment 2
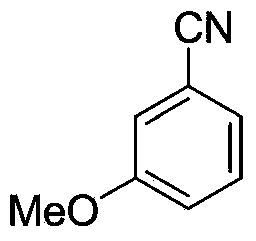
[0035] Synthesis of m-methoxybenzonitrile
[0036] Under the protection of nitrogen, add 61mg (ie 0.5mmol) m-methoxytoluene, 103mg (ie 1.0mmol) nitroso tert-butyl ester, 25mg (0.15mmol) N-hydroxyphthaloyl to the microwave tube Amine and 5.6 mg of palladium acetate (ie 0.025 mmol) were heated to 70° C. in 0.5 mL of acetonitrile to react for 24 hours. After the reaction system was cooled to room temperature, 2 mL of dichloromethane was added for dilution. The mixture was filtered and concentrated. The reaction solution is purified by column chromatography with sherwood oil as an eluent to obtain m-methoxybenzonitrile, and its structure is shown in the following formula:
[0037]
[0038] This compound is colorless oily liquid, and productive rate is 81%, and its data are as follows:
[0039] 1 H NMR (CDCl 3 ,400MHz):δ=7.39-7.35(m,1H),7.28-7.23(m,1H),7.14-7.13(m,2H),3.83(s,3H); 13 C NMR (CDCl 3,100MHz): δ=159.5,130.2,124.4,119.2,118.6,116.8,113.1,55.4.
Embodiment 3
[0041] Synthesis of o-methoxy tert-butyl ester
[0042] Under the protection of nitrogen, add 61mg (ie 0.5mmol) of o-methoxytoluene, 154mg (ie 1.5mmol) of nitroso tert-butyl ester, 25mg (0.15mmol) of N-hydroxyphthaloyl to the microwave tube Amine and 5.6 mg of palladium acetate (ie 0.025 mmol) were heated to 70° C. in 0.5 mL of acetonitrile to react for 24 hours. After the reaction system was cooled to room temperature, 2 mL of dichloromethane was added for dilution. The mixture was filtered and concentrated. The reaction solution is purified by column chromatography using petroleum ether as an eluent to obtain o-methoxybenzonitrile, and its structure is shown in the following formula:
[0043]
[0044] This compound is yellow liquid, and productive rate is 92%, and its data are as follows:
[0045] 1 H NMR (CDCl 3 ,400MHz):δ=7.57-7.53(m,2H),7.03-6.98(m,2H),3.93(s,3H); 13 C NMR (CDCl 3 ,100MHz): δ=161.0,134.3,133.5,120.6,116.4,111.2,101.5,55.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com