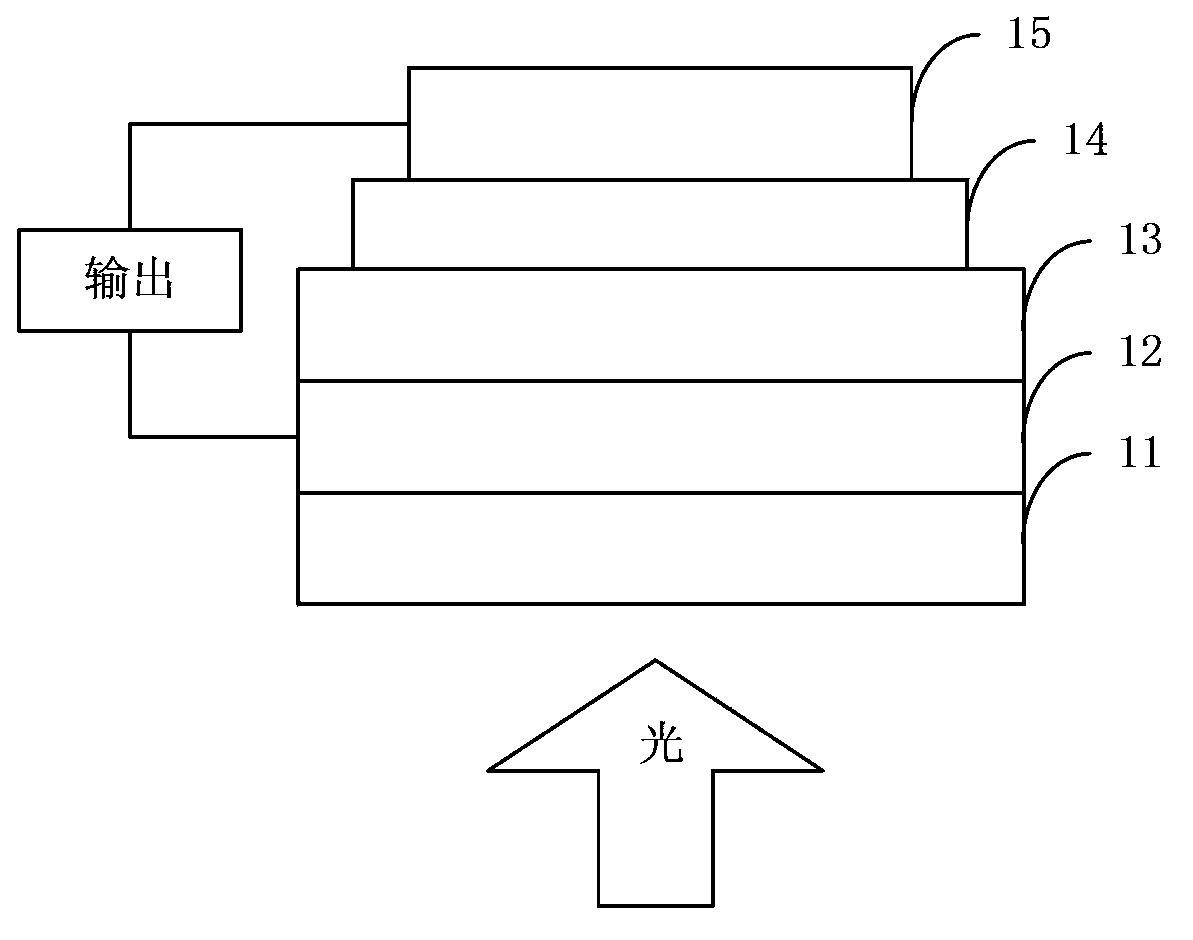
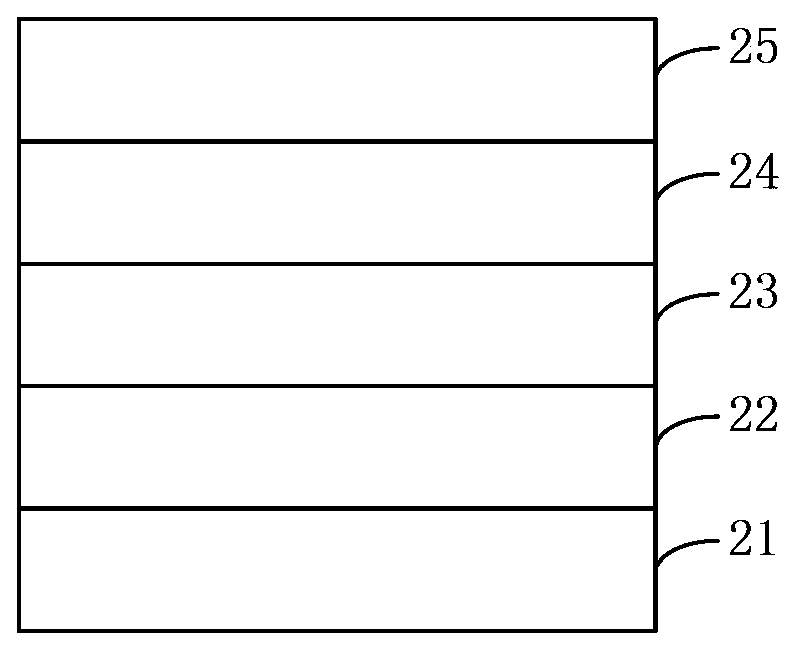
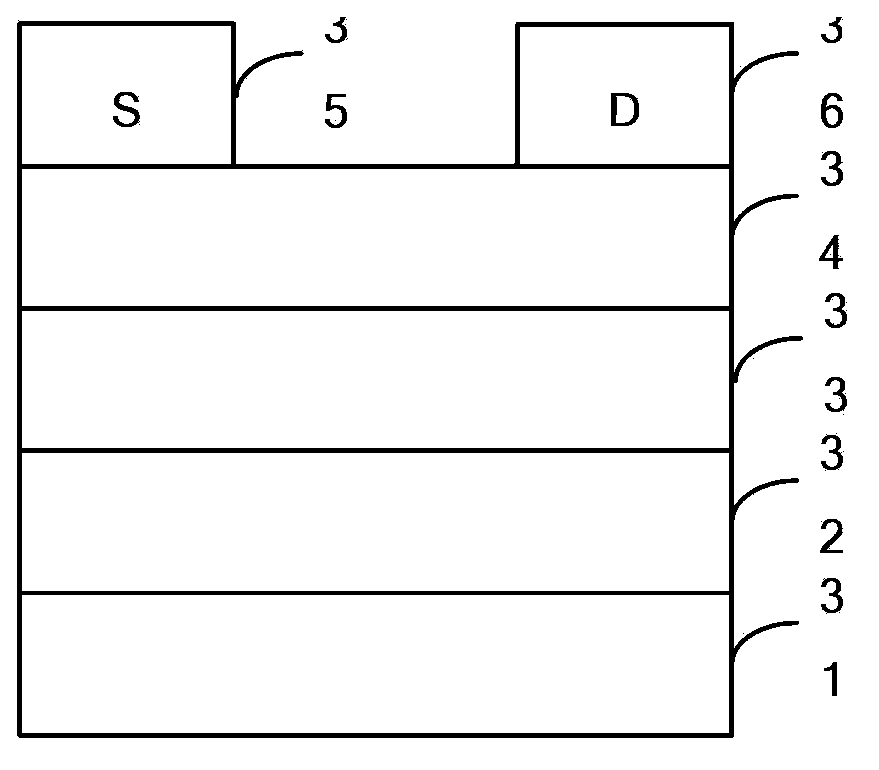
Dibenzothiophene copolymer containing diazosulfide unit as well as preparation method and application of dibenzothiophene copolymer
A technology of benzodithiophene and benzothiadiazole, which is applied in the field of benzodithiophene copolymer and its preparation, can solve the problems of low photoelectric conversion efficiency and the like, and achieves improved carrier mobility, high yield, mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The preparation method of the above-mentioned benzodithiophene copolymer containing benzothiadiazole unit comprises the following steps:
[0033] S1, the structural formula is Compound A (that is, thiophene acetic acid derivatives, for R 1 , R 2 The difference of the substituent, the chemical nomenclature in each embodiment will be different, see each embodiment for details) use dichloromethane (CH 2 Cl 2 ) after dissolving, a mixed solution was obtained, and in an oxygen-free environment (at least one of nitrogen and argon exists), the mixed solution was added dropwise to 1,3-dicyclohexylcarbodiimide (DCC) and 4- The reaction is carried out in dichloromethane in the presence of dimethylaminopyridine (DMAP). After the reaction is stopped, the structural formula is Compound B (i.e. thiophene acetone derivatives, for R 1 , R 2 Different substituents, the chemical names in each embodiment will be different, see each embodiment for details);
[0034] Wherein, the m...
Embodiment 1
[0053] This embodiment discloses a benzodithiophene copolymer containing benzothiadiazole units with the following structure (R 1 =R 2 = H, R 3 = Octyloxy, R 4 = H, R 5 =R 6 = Octyloxy):
[0054] n=60;
[0055] The preparation steps of the above-mentioned benzodithiophene copolymer containing benzothiadiazole units are as follows:
[0056] One, the preparation of 1,3-two (2-thiophene) acetone
[0057]
[0058] First, with 70 mL treated dichloromethane (CH 2 Cl 2) Dissolve 7.6g (36.8mmol) of 1,3-dicyclohexylcarbodiimide (DCC) and 1.23g (10mmol) of 4-dimethylaminopyridine (DMAP) to obtain a mixed solution. Under nitrogen protection, the A solution of 5 g (35.2 mmol) of 2-thiopheneacetic acid in 70 mL of dichloromethane was added dropwise to the mixed solution and reacted overnight. After the reaction, the reaction solution was filtered, and recrystallized twice with n-hexane, and then separated and purified by column chromatography to obtain the structural formula...
Embodiment 2
[0077] This embodiment discloses a benzodithiophene copolymer containing benzothiadiazole units with the following structure (R 1 = Hexyl, R 2 = H, R 3 = methyl, R 4 = H, R 5 =R 6 = tetradecyloxy):
[0078] n=50;
[0079] The preparation steps of the above-mentioned benzodithiophene copolymer containing benzothiadiazole units are as follows:
[0080] 1. Preparation of 1,3-bis(3-hexyl-5-thiophene)acetone:
[0081]
[0082] First, with 70 mL treated dichloromethane (CH 2 Cl 2 ) Dissolve 7.6g (36.8mmol) of 1,3-dicyclohexylcarbodiimide (DCC) and 1.23g (10mmol) of 4-dimethylaminopyridine (DMAP) in the presence of mixed gas of argon and nitrogen , 60 mL of dichloromethane solution dissolved with 8.0 g (35.2 mmol) of 3-hexyl-5-thiopheneacetic acid was added dropwise to the above reaction solution, and reacted overnight. After the reaction, the reaction solution was filtered, recrystallized twice with n-hexane, and then separated and purified by column chromatography to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com