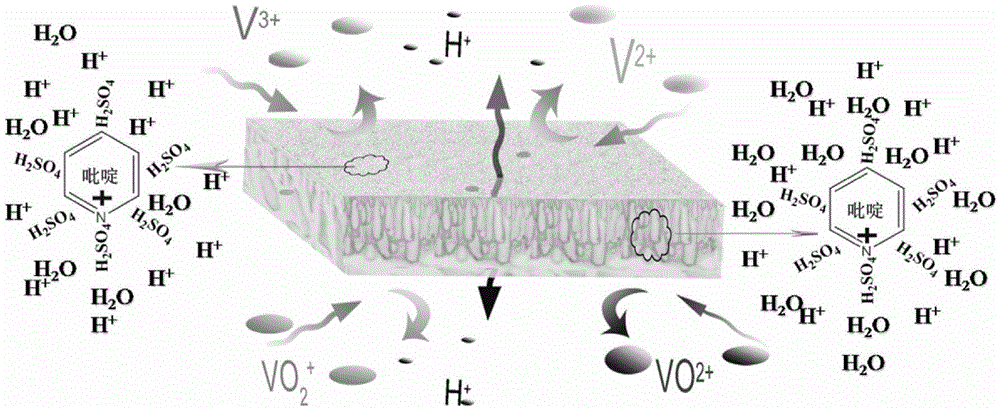
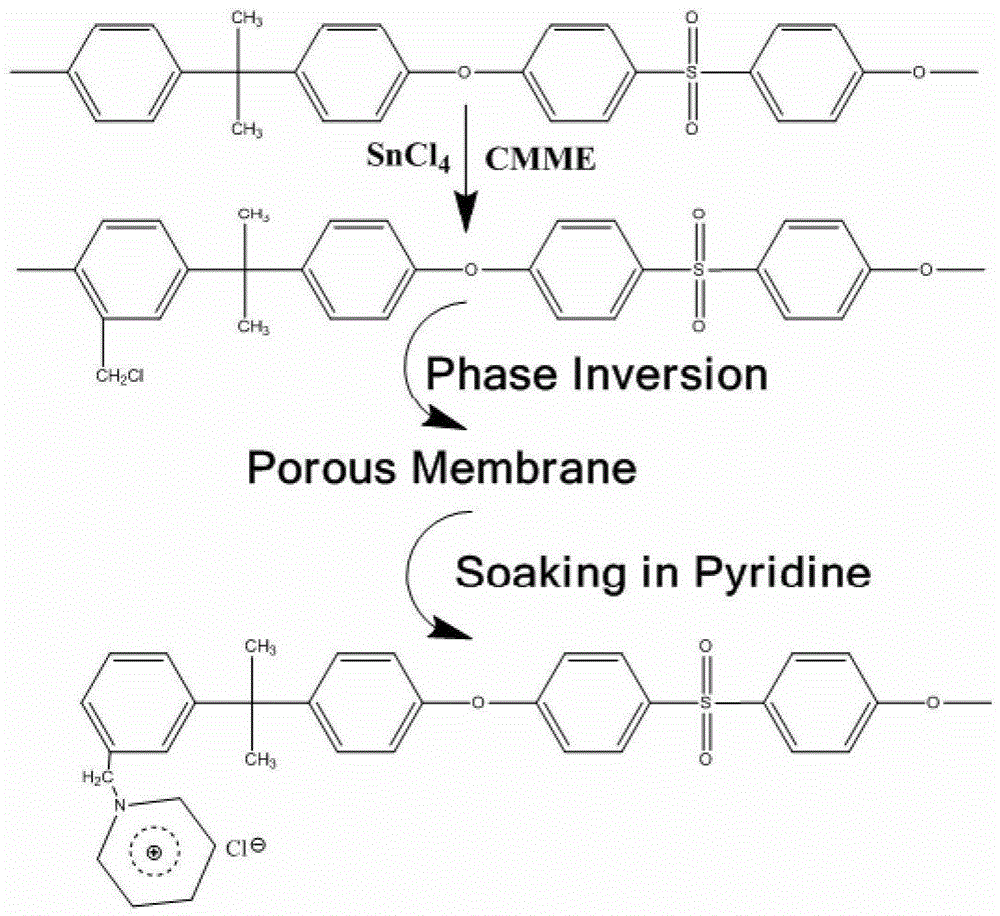
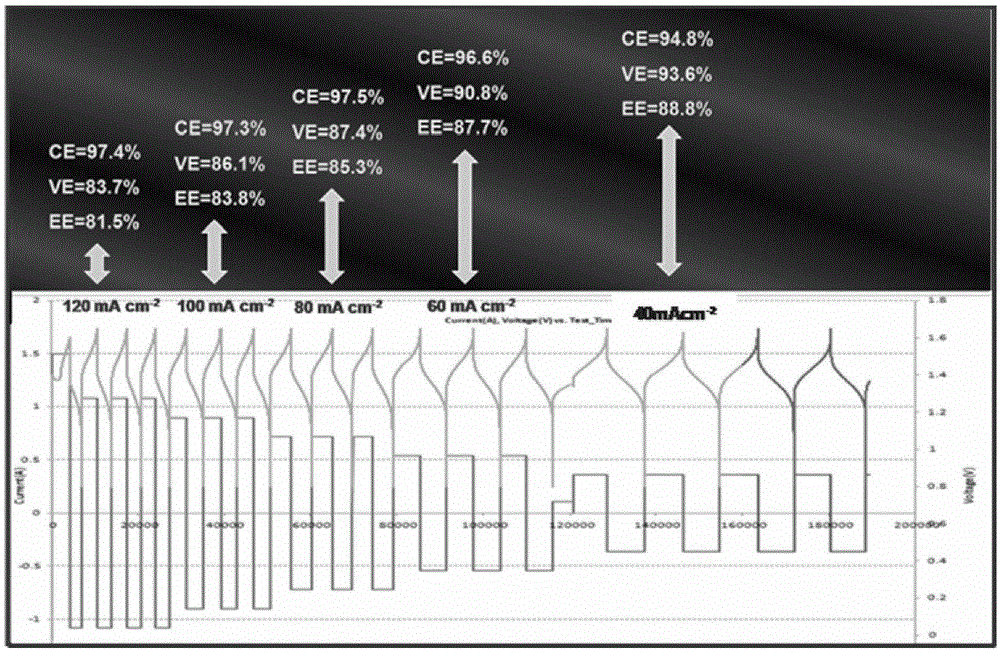
Application of an Alkaline Porous Membrane in Flow Energy Storage Batteries
A liquid flow energy storage battery and porous membrane technology, which is applied to fuel cell parts, fuel cells, battery pack parts, etc., can solve the problems of high price, poor ion selectivity, and limited membrane industrial application, and achieve low cost , high conductivity, and the effect of improving ion selective permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 2g of chloromethyl polysulfone (the degree of chloromethylation is 135mmol / g) is dissolved in 8g of DMAC, stirred for 5 hours, and the polymer solution formed is spread on the surface of a glass plate, and scraped into a thickness of 250um under normal temperature and pressure. liquid film. After 10 seconds, place the glass plate together with the liquid film in a constant temperature and humidity chamber at 50°C with a humidity of 80%, and take it out after 5 minutes to form a porous diaphragm.
[0039] The prepared porous diaphragm was soaked in deionized water for 24 hours, and then immersed in a solution of pyridine:water=1:3 (volume ratio) for 12 hours. Afterwards, the porous membrane was washed with deionized water, and immersed in 3mol / L sulfuric acid aqueous solution for 1 hour to obtain a porous composite membrane. The cross section and surface structure of the membrane were as follows: Figure 4 , 5 shown. Depend on Figure 4 , 5 It can be seen that the e...
Embodiment 2
[0045] 1g of chloromethyl polysulfone (the degree of chloromethylation is 135mmol / g) is blended with 1g of ordinary polysulfone, dissolved in 8g of DMAC, stirred for 24 hours, and the resulting polymer solution is spread on the surface of a glass plate and kept at room temperature. Press down and scrape into a liquid film with a thickness of 250um. After 10 seconds, place the glass plate together with the liquid film in a constant temperature and humidity chamber at 50°C with a humidity of 80%, and take it out after 5 minutes to form a porous diaphragm.
[0046] The prepared porous diaphragm was soaked in deionized water for 24 hours, and then immersed in a solution of pyridine:water=1:9 (volume ratio) for 24 hours. Afterwards, the porous membrane was washed with deionized water, and immersed in 3 mol / L sulfuric acid aqueous solution for 24 hours.
[0047] The all-vanadium flow energy storage battery is assembled by using the prepared porous membrane, in which the catalytic l...
Embodiment 3
[0049] 1g of bromomethylated polysulfone (the degree of bromomethylation is 100mmol / g) was stirred for 15 hours, and the formed polymer solution was spread on the surface of a glass plate, and then quickly immersed in 5L of water to solidify to form a porous diaphragm.
[0050] The prepared porous diaphragm was soaked in deionized water for 24 hours, and then immersed in a solution of imidazole:water=1:3 (volume ratio) for 24 hours. Afterwards, the porous membrane is washed with deionized water and immersed in 3 mol / L sulfuric acid aqueous solution for 24 hours to obtain an alkaline porous membrane containing imidazole groups.
[0051] The all-vanadium flow energy storage battery is assembled by using the prepared porous membrane, in which the catalytic layer is activated carbon felt, the bipolar plate is graphite plate, and the effective area of the membrane is 9cm -2 , the current density is 160, 140, 120, 80, 60, 40mAcm -2 , the vanadium ion concentration in the electrol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com